Since June last year, patent proprietors have had the option of registering their European patents as Unitary Patents. Instead of having a bundle of national rights, proprietors have the option to maintain a single Unitary Patent right extending across the member states participating in the Unified Patent Court (UPC).
The primary attraction of such Unitary Patents is the lower cost for patent protection, as protection can be maintained across 17 participating member states by filing a single translation with the European Patent Office (EPO) and paying maintenance fees similar to the costs of maintaining protection in four of those countries. The major downside of such Unitary Patents is that they are always subject to revocation in a single court action, whereas if opted-out from the jurisdiction of the UPC, individual national patents can only be revoked on a country-by- country basis.
The EPO has been tracking the uptake of Unitary Patents and according to the EPO website patent proprietors are choosing to convert around 18% of European patents into Unitary Patents. The EPO also reports both the technology fields where the greatest numbers of Unitary Patents have been granted and the patent proprietors owning the greatest numbers of Unitary Patents.
As of 1 March 2024, the EPO reported the following as the top ten fields for Unitary Patents and the top ten proprietors of Unitary Patents.
| Technology Field | UPs | |
|---|---|---|
| 1 | Medical Technology | 2524 |
| 2 | Civil Engineering | 1267 |
| 3 | Transport | 1164 |
| 4 | Other machines | 1142 |
| 5 | Measurement | 1117 |
| 6 | Digital Communications | 1095 |
| 7 | Electrical Machinery | 1022 |
| 8 | Computer Technology | 919 |
| 9 | Handling | 851 |
| 10 | Pharmaceuticals | 782 |
| Technology Field | UPs | |
|---|---|---|
| 1 | Johnson & Johnson | 364 |
| 2 | Siemens AG | 340 |
| 3 | Qualcomm Inc. | 264 |
| 4 | Samsung | 258 |
| 5 | L M Ericsson | 241 |
| 6 | Volvo | 194 |
| 7 | Becton, Dickinson & Co. | 143 |
| 8 | Huawei | 128 |
| 9 | Fraunhofer Gesellschaft | 122 |
| 10 | Vestas | 110 |
From the raw numbers, it would appear that the Unitary Patent System has been enthusiastically embraced by tech giants such as Siemens, Qualcomm, Samsung and Ericsson which has led to digital communications being the 6th most common field of technology for Unitary Patents. However, such raw numbers do not take account of the fact that there are far higher numbers of patent applications in some fields than others. When this is considered, a very different picture appears.
Take up of Unitary Patents by field of technology
Scaled by numbers of patent applications in each field of technology, the take up of Unitary Patents in different fields to date are as follows:
Technology fields where Unitary Patents are most popular
Technology fields where Unitary Patents are least popular
| (%) Unitary | ||
|---|---|---|
| 1 | Civil Engineering | 40.0% |
| 2 | Furniture | 30.0% |
| 3 | Machine Tools | 27.7% |
| 4 | Handling | 27.5% |
| 5 | Other Machines | 25.9% |
| 6 | Thermal Processes | 24.5% |
| 7 | Medical Technology | 24.4% |
| 8 | Mechanical Elements | 23.5% |
| 9 | Environmental Technology | 23.5% |
| 10 | Chemical Engineering | 23.3% |
| (%) Unitary | ||
|---|---|---|
| 1 | IT methods | 6.4% |
| 2 | Semiconductors | 7.3% |
| 3 | Polymers | 9.2% |
| 4 | Audio visual | 9.2% |
| 5 | Computers | 9.8% |
| 6 | Biotechnology | 9.9% |
| 7 | Digital Communications | 10.9% |
| 8 | Electrical Machinery | 12.4% |
| 9 | Materials Chemistry | 12.9% |
| 10 | Organic Chemistry | 12.9% |
Similarly, when compared with the numbers of patent applications various proprietors filed in 2021 (a reasonable indicator of the numbers of patent applications filed by an applicant each year), the extent to which some of the top Unitary Patent proprietors have embraced Unitary Patents is more tempered as is shown in the table below. As can be seen, in reality, Samsung and Huawei are actually converting a relatively small proportion of their patent applications into Unitary Patents.
| Proprietor | Unitary Patents | 2021 Applications | Ratio | |
|---|---|---|---|---|
| 1 | Johnson & Johnson | 364 | 861 | 42% |
| 2 | Siemens AG | 340 | 1720 | 20% |
| 3 | Qualcomm Inc. | 264 | 1534 | 17% |
| 4 | Samsung | 258 | 3439 | 8% |
| 5 | L M Ericsson | 241 | 1884 | 13% |
| 6 | Volvo | 194 | 466 | 42% |
| 7 | Becton, Dickinson & Co. | 143 | 294 | 49% |
| 8 | Huawei | 128 | 3544 | 4% |
| 9 | Fraunhofer Gesellschaft | 122 | 564 | 22% |
| 10 | Vestas | 110 | 154 | 71% |
Rather, when considering the numbers of patent applications different applicants file, the most enthusiastic users of the Unitary Patent System relative to patent filings appearing in the EPOs top users list, are Align Technology, followed by Pirelli and Vestas (see below).
| Proprietor | Unitary Patents | 2021 Applications | Ratio | |
|---|---|---|---|---|
| 1 | Align Technology | 50 | 16 | 313% |
| 2 | Pirelli | 49 | 38 | 129% |
| 3 | Vestas | 110 | 154 | 71% |
| 4 | Becton, Dickinson & Co. | 143 | 294 | 49% |
| 5 | SEB | 50 | 103 | 49% |
| 6 | Johnson & Johnson | 364 | 861 | 42% |
| 7 | Volvo | 194 | 466 | 42% |
| 8 | Tata Group | 95 | 228 | 42% |
| 9 | Philip Morris | 106 | 271 | 39% |
| 10 | L’Oreal | 100 | 353 | 28% |
Notable also are the major patent filers who appear on the EPOs top filers lists, but who have made very little or no engagement with the Unitary Patent System as are listed below.
| Proprietor | Unitary Patents | 2021 Applications | Ratio | |
|---|---|---|---|---|
| 1 | LG | 25 | 2422 | 1% |
| 2 | Raytheon | 0 | 1623 | 0% |
| 3 | Sony | 0 | 1465 | 0% |
| 4 | Robert Bosch | 22 | 1289 | 2% |
| 5 | Microsoft | 0 | 1211 | 0% |
| 6 | OPPO Mobile | 12 | 1057 | 1% |
| 7 | Nokia | 26 | 1038 | 3% |
| 8 | Alphabet | 0 | 1023 | 0% |
| 9 | General Electric | 2 | 871 | 0% |
| 10 | Hitachi | 22 | 774 | 3% |
Other, notable opt-outs appearing in the EPO’s top 50 patent filers for 2021, who appear yet to have engaged with the Unitary Patent system include: Baidu, 3M, CEA, Dow Chemical, HP and NTT Docomo, all of whom filed between 691 and 461 European patent applications in 2021 and all of whom are yet to register any Unitary Patents.
Conclusions
The picture these numbers paint is a nuanced one.
Historically, around 50% of European patent applications (most typically electronics patent applications) were validated and maintained in UK, Germany and France. A further 40% (often mechanical patents) were maintained in between 4-6 jurisdictions (often UK, Germany and France and along with a selection from Italy, Spain, and the Netherlands) with the remaining 10% of European patents (typically pharmaceutical patents) being maintained more broadly, sometimes (around 2% of patents) much more broadly.
That the fields of technology where proprietors most frequently choose Unitary Patents (e.g. civil engineering, furniture, machine tools, handling & other machines) are predominately in the mechanics field, suggests that to date, the success of the Unitary Patent has been replacing European patents which previously would have been maintained moderately broadly. Presumably, patent proprietors in such fields have been attracted by the potentially lower maintenance costs for such patents.
It is, however, also clear that different patent proprietors, even in the same areas of technology have very different approaches. Some (e.g. Volvo and Pirelli in the automotive field) are enthusiastic users of the new system. In contrast other major filers in the same field (e.g. Volkswagen who filed 459 European patent applications in 2021 and currently have 13 Unitary Patents) have barely used it.
Moving on to the electronics fields, the numbers suggest that some proprietors (e.g. Siemens and Ericsson) are choosing to covert around 15-20% of their European patents into Unitary Patents. However, many others (e.g. Samsung, Huawei & Nokia) are choosing to obtain Unitary Patents at a far lower rate or (e.g. Sony, Microsoft, NTT Docomo) not at all.
The relatively low take up of Unitary Patents in the electronics field relative to the total numbers of patent applications filed in that field suggests that the additional translation costs involved in registering a Unitary Patent compared with maintaining rights solely in UK, Germany and France (where protection can be obtained without filing a translation of a patent) is causing proprietors in those fields to delay embracing the Unitary Patent.
For large companies, a selective approach is entirely sensible. Typical translation costs for a Unitary Patent are expected to be around €5,000 per patent and translation costs will mount up to a considerable sum when a patent proprietor is filing thousands of applications each year.
As the present analysis focuses on the most active users of the Unitary Patent System and the largest filers of European patent applications, the behaviour of smaller companies is harder to discern. However, there are hints that at least some smaller companies are actively embracing the Unitary Patent System. Many of the fields of technology where Unitary Patents are proving popular (e.g. civil engineering, furniture, machine tools, handling & other machines) are fields which tend not to be dominated by exceptionally large filers of patent applications. It is also notable that it is the two smallest patent filers (Align Technology and Pirelli) covered by this study, both of whom filed less than 50 patent applications in 2021, who appear to be converting the greatest proportions of their patent portfolios into Unitary Patents.
Finally, the relatively low take up of Unitary Patents in the chemical and life science fields, would seem to confirm the reluctance of patent proprietors or at least the most frequent patent applicants, in those fields to expose potentially very valuable individual patent rights to the risk of revocation in a single court action. In most cases, this reluctance would appear to outweigh the potentially significant cost savings which the Unitary Patent route affords rights which are broadly maintained across Europe. However, even then, patent proprietors in those fields are converting around 10-15% of their granted European patents into Unitary Patents.
Commentary by Partner Max Thoma has been featured in Accountancy Age, International Tax Review and World Intellectual Property Review, discussing how making the Patent Box relief more generous would act as an incentive for domestic firms to invest more in research and development (R&D).
Read the extended press release below.
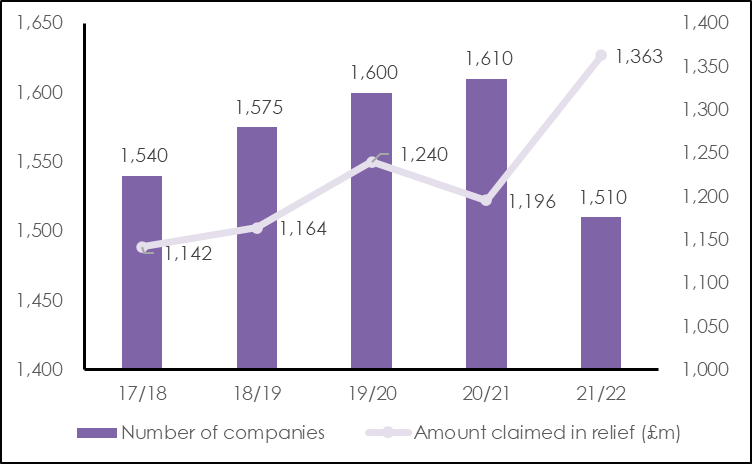
The amount of tax saved by businesses through HMRC’s Patent Box scheme has increased by 23% over the last five years, from £1.14 billion in 2017/18 to £1.4 billion in 2021/22, says leading intellectual property (IP) firm Mathys & Squire.
The Patent Box, introduced in 2013, is a tax incentive that allows UK businesses to pay just 10% corporation tax on profits derived from any UK or EU patents. It was introduced by the Government to encourage businesses to invest more in research and development (R&D).
With Corporation Tax now at 25% that tax break has become increasingly attractive.
Max Thoma, Partner at Mathys & Squire says that making the Patent Box relief more generous would act as an incentive for domestic firms to invest more in R&D. This is possible now that the UK is no longer a member of the European Union and bound by its state aid restrictions.
The Patent Box tax relief is seen as one way to help improve the UK’s low level of R&D spend. Overall spending on R&D in the UK is estimated to be 2.9% of GDP*. This trails behind countries such as the US (3.47% of GDP), Japan (3.27%) and Germany (3.13%).
Says Max Thoma: “The Patent Box rules have had a positive effect on the amount British businesses invest in R&D. However, we still lag behind some of our global competitors – an even better Patent Box would help to close that gap.”
Additionally, the UK lags significantly behind other G7 countries in terms of the total number of patent applications. In 2022, applications to the IPO reached 19,500**- however this is dwarfed by the number of patents filed by applicants based in China (1.58 million), the United States (505,000) and Japan (405,400)***.
Despite the rising amount of relief being provided through the Patent Box system, the number of companies claiming relief has fallen from 1.540 in 2017/18 to 1,510 in 2021/22. Mathys & Squire point out that this could be a sign that the Patent Box scheme is much less well known than R&D credits.
Sectors including construction (1% of Patent Box claims) and IT (4% of claims) make very limited use of the Patent Box scheme, suggesting that businesses in these sectors are missing out of opportunities to both protect their IP and reduce their corporation tax bills.
Says Max Thoma: “A significant number of companies may not be aware of the benefits of the Patent Box or that they could be claiming a significant tax relief.”
“Construction and IT are both sectors where businesses are not always aware that they may be able to patents systems and techniques they have developed. Some are paying too much tax as a result.”
Change in number of companies claiming Patent Box relief and amount of relief claimed (in £m)
*World Bank
**IPO
*WIPO
This year for International Women’s Day, we asked a few of our partners to highlight inspiring female inventors and pioneers that have had a lasting impact on intellectual property (IP). Partners Jane Clark, Helen Cawley, Caroline Warren and Dani Kramer outline the incredible careers and legacies of Sheila Lesley, Kathi Vidal, Anna Connelly and Grace Hopper.
Partner Jane Clark recounts her experience with Sheila Lesley
Partner Jane Clark recounts her experience with Sheila Lesley OBE, one of the ground-breaking examples in the UK of women in IP and the first female President of CITMA, The Chartered Institute of Trade Mark Attorneys.
“My female role model in IP, well there weren’t many around in the UK back then, but in any event it has to be the incomparable Sheila Lesley. Sheila was a pioneer for women in the UK IP profession.”
As set out in the linked obituary, Sheila attended Girton College, Cambridge, specialising in Natural Sciences and Law but decided not to pursue a career in chemical research after being inspired by seeing John Logie Baird, the inventor of television, walking across the golf course in Bude. Instead, Sheila joined Forrester Ketley & Co. (now Forresters IP) qualifying as a UK Chartered Patent Agent (as UK Patent attorneys were then known) in 1953.
“Ours was then a male-dominated profession, so much so that Sheila was the first woman in 29 years to qualify as a UK Chartered Patent Agent (as UK Patent attorneys were then known)!”
Sheila went on to become the first female president of The Chartered Institute of Trade Mark Attorneys and her contributions were recognized with an OBE in 1988.
“I started work as a trainee patent attorney at what was then Forrester Ketley & Co. when Miss Lesley (as we were expected to call her) was already a senior partner. As a senior partner of the firm, she was employing far more women trainees than any other patent attorney firm in the UK at that time.”
“Despite the fact that Miss Lesley focused on trade marks, she always found time to support the female trainee patent attorneys. Indeed, I vividly recall taking a general inquiry call from a “gentleman” who said to me he didn’t want to talk to a girl and would I put him through to a man. I was polite but obviously offended, explained to the “gentleman” that I would transfer the call to one of our senior partners and transferred the call to Miss Lesley explaining before I transferred the call exactly what the “gentleman” had said. Miss Lesley told me later that she had politely told the “gentleman” concerned that we did not want his business. I would still love to know exactly what Miss Lesley said to him!”
Partner Helen Cawley showcases the work of Kathi Vidal
Partner and Head of Trade Marks, Helen Cawley, wanted to highlight Kathi Vidal, Under Secretary of Commerce for Intellectual Property and Director of the United States Patent and Trademark Office (USPTO).
“Kathi is clearly a very intelligent lady who is at the top of her profession. At the same time as being a role model she invests time in others to help them reach their full potential.”
As chief executive of the USPTO, Kathi Vidal heads one of the largest Intellectual Property (IP) offices in the world, with an annual budget of $4 billion and over 13,000 public servants. Kathi was named as one of Managing IP’s top 50 most influential people in IP in 2022.
Attending Binghamton University at the age of 16, Kathi received a bachelor’s in electrical engineering and accepted a position at General Electric Aerospace, (now Lockheed Martin), prior to graduating. During this time her time at General Electric Aerospace, she designed one of the first A.I. intelligence systems for aircraft, as well as aircraft engine-control systems that are still used today.
She has helped to protect intellectual property rights, representing a broad spectrum of companies from start-ups with limited resources to some of the world’s most well-known companies.
Throughout her career, Kathi has shone a light on the importance of mentoring and creating opportunities for women from diverse backgrounds. She continues this important work today.
Partner Caroline Warren outlines Anna Connelly’s lasting impact on public safety
Partner Caroline Warren wanted to celebrate Anna Connelly, a famous innovator that developed an external metal staircase in 1887, which is considered to be one of the earliest systems specifically designed as a fire escape.
One of America’s earliest female patentees, Anna Connelly’s contributions to public safety has seen her responsible for saving thousands of lives for over a century. She was one of the first women to be granted a patent for an invention after the American Civil War, and revolutionised building safety through her system of exterior metallic staircases and platforms that enabled people to escape a building in the case of a fire.
“Anna’s invention is one of those ideas that seems so obvious after it’s been thought of, but it was a real revolution at the time and has saved countless lives since.”
An extract from Anna Connelly’s original patent (US368816A) describes the logic behind her ingenious design:
“My invention relates to improvements in fire-escapes; and it consists of a bridge surrounded by a railing and having openings in the ends of the floor thereof, as herein described, the said bridge being adapted to be placed on the roofs of adjoining or adjacent buildings, thereby permitting the ready and safe passage from one roof to the other.”
Partner Dani Kramer highlights Grace Hopper’s legacy in computer programming
Partner Dani Kramer wanted to showcase the incredible work of Grace Hopper, a pioneer in computer programming.
“The trailblazing contributions that Grace Hopper made to the early development of computer programming languages and the compiler led to the computing industry as we now know it. “
In 1952, Hopper developed the first ‘compiler’, a computer program that allows written instructions to be translated into computer code.
“What I was after in beginning English language [programming] was to bring another whole group of people able to use the computer easily … I kept calling for more user-friendly languages. Most of the stuff we get from academicians, computer science people, is in no way adapted to people,” Hopper explained in a 1980 interview.
Born in 1906, Grace Hopper graduated from Vassar College with degrees in mathematics and physics, later joining the US Navy following the bombing of Pearl Harbour. She was assigned to the Bureau of Ships computation project at Harvard following an initial rejection due to her age and diminutive size, according to a Yale University biography. This project saw her work on the Mark I, the first US electromechanical computer, calculating rocket trajectories, anti-aircraft gun range tables and calibrating minesweepers.
After the war, she joined the Eckert-Mauchly Computer Corporation, later Sperry Rand, where she pioneered the idea of automatic programming. It was here that she developed the compiler.
Nvidia, the global market leader in computer chips used in AI applications, and CEO Jensen Huang, recognise the importance of Hopper to AI computing and named one of their latest chips after her – the GH200 Grace Hopper Superchip. The company’s H100 chips (H for Hopper) are specifically designed for AI applications, and power generative AI services like ChatGPT.
On 6 March 2024, Mathys & Squire sponsored and attended the inaugural Non-Law Into Law Conference at the London School of Economics (LSE). The event brought together (and was co-organised by) students and law societies from LSE, Imperial College London, University College London, Queen Mary, Warwick University and Durham University, alongside firms from across the legal field.
Partner James Pitchford and Technical Assistant Louis Brosnan ran an intellectual property (IP) workshop in which the attendees worked in groups to tackle different challenging scenarios. Participants were encouraged to first think like an inventor and then as patent and trade mark attorneys – to devise solutions to real-world problems and then analyse the ways in which the innovative elements of their ideas could be protected as valuable IP assets.
Later in the evening, Technical Assistants Louis Brosnan and Annabelle Carver participated in a panel discussion on joining law from a non-law background. They sat alongside trainees from Linklaters, Slaughter and May and Jones Day and discussed questions relating to navigating the interview process and the fresh perspective that a non-law degree can bring to the industry. Louis and Annabelle provided insights on topics such as getting the most out of training sessions, how Mathys & Squire promotes diversity & inclusion in the workplace, and our approach to environmental considerations and social responsibility. They also discussed in detail what the day-to-day job of a patent attorney entails, and how the cooperative working environment at Mathys & Squire really makes a difference to those stepping onto the first rung of their career ladders.

Left to right: Technical Assistant Annabelle Carver, Partner James Pitchford and Technical Assistant Louis Brosnan of Mathys & Squire
Mathys & Squire is proud to continue fostering the development of those interested in pursuing a career in IP.
A three-day hearing began on Monday 19th February to address an appeal against the initial judgment which concluded that Tesco had infringed Lidl’s trade marks and copyright. Additionally, Lidl has appealed the decision to revoke one of its trade marks.
The Court of Appeal is tasked with determining whether the High Court’s finding that Tesco’s ‘Clubcard Prices’ logo infringed upon Lidl’s logo, both featuring a yellow circle within a blue square (Fig.1), was correct.

This case covers a range of intellectual property rights, including passing off, trade marks, and copyright. The anticipated decision of the Court is expected to provide clarity on various aspects, notably claims of ‘evergreening’ and issues of bad faith.
Should Tesco’s appeal prove unsuccessful, it will be required to remove the Clubcard logo which has become very familiar across its stores.
As the hearing has now concluded, we eagerly await the decision.
See our previous coverage of the case here.
Mathys & Squire is proud to have worked with MS-RT – an offshoot of Ford’s rally partner M-Sport – in protecting their IP as they redefine the landscape of commercial vehicle design with the introduction of the all-new Ford Transit Custom MS-RT and Ford Ranger MS-RT.

Developed by MS-RT in partnership with Ford Pro, these new MS-RT models seamlessly blend Ford Motor Company’s renowned durability with motorsport-inspired design innovations. The launch has received huge praise across the automotive industry, with over 200 individual articles posted within the first 24 hours of the launch. The new models are available to order from Ford Pro dealers across Europe, with cars expected on the road in mid-2024.
Working closely with MS-RT’s design and legal team, Mathys & Squire prepared and filed a comprehensive set of design registrations meticulously covering various aspects of MS-RT’s innovative designs in various formats to provide a broad scope of design protection, ensuring that the distinctive features of the MS-RT vehicles remain exclusive to the brand.
Adam Gilbertson, Associate at Mathys & Squire, says:
‘For a company like MS-RT whose business and USP is all about its unique designs that transform the look of these vehicles, and which get so much attention, protecting their design IP is critical as the business continues to grow. We are delighted to be part of MS-RT’s journey and see the launch go so well.’

Carolyn Mills, Legal and Contracts Director at MS-RT, says:
‘Having the expert guidance of the Mathys & Squire team to steer us through the process and set a filing strategy has been extremely reassuring.’

Joe Pace, Managing Director at MS-RT, says:
‘Having invested a significant amount of time and money into the development these designs, we felt it was essential to protect this investment with proper design protection. Many thanks to the Mathys and Squire team for all your help with registering our designs for the MS-RT Custom and Ranger. Great work and excellent support throughout the whole project.‘
Mathys & Squire has a dedicated and experienced designs team which has helped numerous household names secure strategic protection for their designs internationally. Find out more about our design services here and contact us here.
An article by Technical Assistant Rebecca Bennett and Partners Anna Gregson and Andrew White outlining the available protection for AI-driven bioinformatics has been featured in the latest edition of The Patent Lawyer.
Read the feature below.
The bioinformatic landscape has undergone a profound shift with the infusion of artificial intelligence (AI) technologies. The integration of AI, and specifically its subsets like machine learning and deep learning, has catalysed a revolution in bioinformatics. This transformative impact is most evident in the enhanced analysis and interpretation of biological data, unlocking invaluable insights from expansive datasets. The synergy between AI and bioinformatics is not only expediting drug discovery but also enriching our understanding of complex biological systems. Today, AI stands as an indispensable instrument within the bioinformatics toolkit, propelling the field into new realms of discovery and innovation.
However, a prevalent misconception has persisted, particularly in Europe, that bioinformatic inventions are inherently unpatentable due to their classification as mathematical methods or computer programs by the European Patent Office (EPO) and as such fall within excluded subject matter (Article 52 European Patent Convention (EPC)). This has led to the belief among inventors that their innovations may be ineligible for patent protection. While it is true that mathematical methods and computer programs, in isolation, are excluded from patentability, it is essential to recognise that many AI-driven inventions, particularly those in the dynamic field of bioinformatics, do not fit this restrictive narrative and are not classed as excluded subject matter.
This misconception is evidently mirrored in the discernible contrast in the number of patent applications across various jurisdictions. In 2022, both the United States and China saw a substantial surge in bioinformatics-related patents, with over 4000 published patent applications each. In stark contrast, Europe recorded roughly half that number, totalling just over 2000 published patent applications in the same year. This marked divergence in patent activity may be attributed, in part, to another prevalent misperception — the belief that the United States is more permissive when it comes to software-related inventions, such as those at the intersection of bioinformatics and AI implementation. It’s essential to note that while the United States Patent and Trademark Office (USPTO) may have exhibited a historically more permissive stance toward software-related innovations, including those in bioinformatics and AI, the landscape has significantly evolved in the past decade. A discernible shift towards a more rigorous approach has emerged.
The way that many AI-related bioinformatics inventions are assessed for patentability in Europe is often under the guise of inventive step. In performing this assessment, when considering whether any features contribute to the presence of an inventive step, the EPO will assess whether those features are “technical” or provide a technical contribution in the sense that they provide a technical solution to a technical problem. There is no explicit definition of what is considered “technical” or not, rather this is framed by case law of the EPO Boards of Appeal built up over the last few decades.
It is often in this domain where we find that the argument on patentability can be won or lost. To support arguments on inventive step, it is helpful to be able to refer to statements in the application as originally filed of technical advantage associated with any features that are being argued over. Therefore, care should be taken at the time of drafting to gain input from a European practitioner, experienced and knowledgeable on EPO case law, as to what may help support the presence of an inventive step and therefore the patentability of the invention in Europe. Adding in statements in a letter of response to the EPO is often not as effective as including such statements in the application as originally filed.
Helpfully, the EPO offers illustrative instances of AI applications across various technology fields to help explain what may be considered “technical” or not. For instance, utilizing a neural network in a heart monitoring device to identify irregular heartbeats constitutes a clear technical contribution. Similarly, the classification of digital images based on low-level features, such as edges or pixel attributes for images, exemplifies typical technical applications of classification algorithms. Therefore, although this a hurdle that an invention has to overcome, there are clear circumstances and approaches to meet this requirement.
Additional challenges arise when assessing whether a patent application provides an enabling disclosure of an invention. In particular, according to the EPC, a fundamental requirement is that “The European patent application shall disclose the invention in a manner sufficiently clear and complete for it to be carried out by a person skilled in the art.”. This sufficiency criterion gains heightened significance in the context of patents that encompass machine learning technologies. In some cases, this may require the description and or disclosure of training data, if these cannot be generalised in some way that is reproduceable by the skilled person.
As European practitioners working with bioinformatics inventions, we witness first hand compelling instances of successful bioinformatic applications implementing AI culminating in granted patents. A prime illustration of this lies in the field of genetics. The EPO has rendered affirmative judgments on inventions pertaining to computational methodologies for detecting mutations, including Single Nucleotide Polymorphisms (SNPs) and Single Nucleotide Variations (SNVs). Furthermore, the EPO has extended its positive rulings to encompass predictive technologies for phenotypic traits—ranging from symptoms and their severity to disease prognosis—based on viral genotypes. These instances underscore the EPO’s recognition of the technical merit and innovative impact of AI-driven bioinformatic solutions, offering encouragement and assurance to companies operating in these cutting-edge domains.
In conclusion, the prevailing misconceptions surrounding bioinformatics inventions, especially those implementing AI, are often unfounded, as these innovations are indeed patentable in Europe. The notion that they face insurmountable obstacles is often unsupported.
In December 2023, it was announced that the UK Intellectual Property Office (UKIPO) had received permission to appeal the High Court’s ruling in Emotional Perception AI Ltd v Comptroller-General of Patents, an appeal that will be heard on 14 May 2024. The Court of Appeal will therefore be revisiting the question of the patentability of software inventions and will have its first opportunity to comment on the patentability of artificial neural network (ANN)-based inventions.
The ongoing Emotional Perception case has generated significant interest in the artificial intelligence (AI) industry and patent profession alike, at a time when global interest in use and development of AI technology is sky rocketing – indeed, the UK Government recently identified AI as one of the five technologies of tomorrow critical to helping drive future discoveries and economic growth in its recent Science and Technology Framework. Emotional Perception is the first UK patent court case to focus on AI technology, and the importance of the case is underlined by the fact that the UKIPO, immediately following the first instance judgement, changed their practice for examination of ANN inventions – a change that, at least for the time being, effectively removes ANN inventions from the scope of the computer program exclusion and has led to a flurry of AI-related patents being allowed.
Seen by many as a long-awaited breakthrough towards the recognition of AI as a technical field in its own right, eligible for patent protection commensurate with its increasing role in driving innovation worldwide, the first instance judgement is also highly controversial, as it represents a significant divergence from established European Patent Office (EPO) practice and has sparked debate and has polarised views amongst the patent community. If not overturned on appeal, the Emotional Perception case will make the UK a far more favourable patentee-friendly place for patenting AI inventions for the foreseeable future.
The decision at first instance
The invention at the heart of the Emotional Perception appeal relates to a system and method for providing improved media file recommendations to an end user which is implemented using an artificial neural network (ANN) trained in a specific way which is said to align its output more closely to how a human semantically perceives the content of an input file.
Initially, the UKIPO rejected Emotional Perception’s application on the grounds that the invention was excluded from patentability for being a computer program as such. That rejection was then reversed by the High Court which concluded (among other things) that the claimed invention, in so far as it relates to the training and use of a trained ANN, did not involve a computer program at all so did not engage the exclusion, and even if it did, the novel method of identifying and providing files to a user was a sufficient “contribution” and sufficiently “technical” to prevent the invention from being considered a “computer program as such”. It is this decision which is now under appeal.
The Law
Since 2007 the UKIPO have used the test set out in Aerotel Ltd v Telco Holdings Ltd to determine whether an alleged invention relates to excluded subject matter. This test involves the following steps:
- Properly construe the claim.
- Identify the actual contribution (although at the application stage this might have to be the alleged contribution).
- Ask whether it falls solely within excluded matter.
- If the third step has not covered it, check whether the actual or alleged contribution is actually technical.
A key question at the heart of the Emotional Perception case was: what was the actual contribution of the claimed invention? Counsel on both sides accepted that the invention related to an ANN-based system for providing improved file recommendations and that the fundamental insight on which the invention was based was the training of the ANN to analyse physical properties of a file using pairwise comparisons of training files. Once trained the trained ANN could then be used to identify, swiftly and accurately, files from a database which correspond semantically to a target file, and to provide file recommendations to a user device.
The judge concluded that although potentially implemented in software, a software ANN was not operating a program in the traditional sense of a set of instructions provided by a programmer because, rather than processing data on a step-by-step instructional basis, machine learning uses training data to self-learn and reconfigure an ANN to solve a specific problem. No other candidate program was identified, and for this reason the “contribution” was held not to engage the computer program exclusion at all. The judge went on to consider step 4 of the Aerotel test anyway and concluded that the contribution was technical in nature and thus also not a computer program as such, because: (i) the end result of sending the file recommendations to an end user provides a relevant external technical contribution; and (ii) a trained ANN itself can also amount to a technical contribution external of the training it received.
Differences of approach in the UK and the EPO
The UKIPO and EPO’s approaches to the assessment of patentable subject matter diverged in the early 2000s when the EPO changed their practice in PBS Partnership/Pension Benefits T931/95. Following that decision, the EPO adopted an “any hardware” approach whereby if a claim involves the use of technical means (e.g. a computer) or is directed to such technical means it avoids the exclusions. As a result, the EPO do not currently raise excluded subject matter objections to “computer-implemented” inventions unless a claim is directed to a purely abstract concept.
The question of whether or not an invention gives rise to a “technical effect” (i.e. whether or not a claimed invention provides a technical solution to a technical problem) remains part of the EPO’s assessment of patentability. However, rather than being considered as part of an assessment of patentable subject matter, it is considered as part of the EPO’s assessment of inventive step.
For computer-implemented inventions, assessment of inventive step involves an exercise of dissecting a claim into technical and non-technical features. Features which serve a technical purpose and contribute to solving a technical problem are taken into account when determining whether or not an invention involves an inventive step. In contrast, features of a claim which are identified as being non-technical are considered to form part of the background to the “technical problem” that an invention might be said to address and are essentially ignored for the purposes of assessing inventive step. Examples of what are considered technical purposes and processes which enable examiners to assess whether or not particular features are “technical” are set out in the EPO’s Guidelines. If an invention is not considered to address a “technical” problem, then it will be considered to be unpatentable in the EPO.
This approach was explicitly endorsed by the EPO’s Enlarged Board of Appeal in Bentley/Pedestrian Simulation G1/19.
The UK Courts have repeatedly stated that the UKIPO and EPO’s approaches are capable of reconciliation and amount to two different ways to (in principle) arrive at the same result. The first instance Emotional Perception decision, however, puts the two approaches into direct conflict.
First, the High Court’s ruling that ANNs inventions do not engage the computer program exclusion at all will in many cases allow AI-based inventions to pass directly to the inventive step stage and even to grant without any consideration of the technical character of the invention.
In contrast to the EPO, assessment of inventive step in the UK is limited to an assessment of whether or not it would be obvious to a person skilled in the art, using common general knowledge to modify any existing item of prior art to arrive at a claimed invention. All claim features are taken into account as part of this assessment and there is no formal separate assessment of the technical character of a claim at the inventive step stage.
Second, the High Court’s ruling that the end result of providing improved file recommendations provides a relevant external technical effect is in direct conflict with the established jurisprudence of the EPO Boards of Appeal who have consistently refused patent applications relating to recommendation systems and methods for lacking a relevant technical effect because any effects of the recommendations (e.g. playing a song, or requesting further recommendations) depends on the subjective choices of the user (see T 0306/10, T 1869/08, T 1983/18, and more recently T 0183/21).
These decisions underline the EPO’s emphasis on the concepts of technical problem, technical purpose and an objective technical effect. By contrast, there was no consideration by the High Court in the Emotional Perception decision of whether or not the invention served a technical purpose or solved any kind of technical problem, or even whether the output of the ANN was to be used as part of a subsequent technical process. Although the judge acknowledged the subjective effect of the recommendation on the user, he then focused on how the recommendation was generated and concluded that this was sufficient to overcome the “technicality” check in the final step of the Aerotel test. Hence, the judgement supports the notion that a trained ANN, processing data through its nodes in a technical way using logic it has learned itself, fulfils the requirements of the Aerotel test regardless of the purpose and the subsequent use of a machine’s output.
The long term impact of the Emotional Perception decision will depend upon the views of the Court of Appeal.
The conclusion at first instance that the computer program exclusion requires the presence of an identifiable set of instructions provided by a human that a computer is to perform is highly questionable and may well be overturned. However, equally important will be how the Court of Appeal addresses the question of whether the technical purpose or nature of the output of an invention is something which needs to be taken into account when assessing patentability. If it does not, then that would leave the door firmly wide open in the UK to the patentability of a wide range of inventions that leverage AI and machine learning, including those in traditionally non-technical fields such as finance, business and administration which would likely be considered to be unpatentable by the EPO.
That having been said, this second part of the Emotional Perception decision seems to be significantly more grounded in UK precedent. In previous decisions such as Protecting Kids the World Over vs Comptroller and Gemstar v Virgin Media, the UK High Court has considered that transfer of data files is sufficiently “technical” to overcome the final check in the Aerotel test, and that there is therefore no need to consider whether or not the output of an invention has a “technical” purpose.
We can expect the outcome of the appeal sometime in the latter half of 2024.
Commentary by Partner Nicholas Fox has been featured in Managing IP, giving an insight into the implications of the UPC Court of Appeal’s ruling that members of the public cannot have access to court documents without using a professional representative. This story has also been covered in JUVE Patent which also discusses the test case which Mathys & Squire have launched in this area, more details of which are discussed here.
Read more on the subject of the ruling by Partners Nicholas Fox and Alexander Robinson below.
The Court of Appeal of the Unified Patent Court (UPC) has decided that members of the public cannot have access to court documents without going through a professional representative. This will act as a further barrier to public access by imposing additional costs, and compares unfavourably to the practice of other courts. It also raises the prospect that the Court of Appeal could dismiss a pending case on access to court documents without giving a ruling on the core issue of how far the Court’s obligation of public access extends.
This is the upshot of the latest procedural order in the protracted dispute over public access to documents in the Ocado v Autostore litigation at the UPC. In an order dated 8 February 2024 the Court of Appeal interpreted its Rules of Procedure so that the requirement for a “party” to be represented also extends to members of the public when applying for access to documents lodged with the Court, even though such members of the public are not parties to the litigation.
The Court of Appeal had requested submissions on whether the member of the public who made a request to access documents filed in the Ocado v Autostore case needed professional representation. Both Ocado and the member of the public who had made the request submitted that representation was not needed, because a member of the public is not a “party” and so the rules requiring “parties” to be represented did not apply to him.
The Court of Appeal has now taken a decision which diverges from the views of both Ocado and the member of the public. Although the Court states that it “does not consider this requirement [for representation] to be unnecessarily burdensome” it has the undesirable effect of making public access more complex than it needs to be. Members of the public will now need to go to the additional expense of appointing a professional representative just to take care of what should be a simple administrative process.
This compares unfavourably with the practice of the European Patent Office (EPO), where any member of the public can access virtually any document filed with the Office simply by consulting the online register. It also compares unfavourably with the practice of the federal courts in the United States, where all pleadings, motions, memoranda and associated documents are available to anyone via the electronic PACER system. Similarly, public access is available in various European countries such as in England and Wales, where statements of case are available to anyone subject to payment of a small fee, and Sweden, where documents held by the court can be requested by email or post subject to payment of a small fee for large numbers of documents or pages.
The ruling raises an important question in relation to the outcome of the appeal. Ocado had previously raised no objections that a member of the public could request access without having to engage legal representation. However, now that the Court has ruled otherwise, it raises the question of whether the original request for access by the unrepresented applicant should be ruled void. Notably, in the context of the appeal, the Court has ordered that previous submissions by the unrepresented applicant should be disregarded as they were not lodged through a representative. It is not clear why the same logic should not also apply to the applicant’s original request, particularly as the Court of Appeal expressly states that the obligation for legal representation applies equally at first instance and during the appeal process.
Previously, Ocado, who are objecting to the release of documents, have not pursued this point as their stated position was that the obligations for legal representation did not apply to access requests. However, now it is to be expected that the validity or otherwise of the initial application will be brought into question.
The Court of Appeal has granted the member of the public 14 days to appoint a representative and has scheduled a hearing on the substance of the appeal to be held on 12 March 2024.
The Court’s order is not yet available on the UPC website, but its contents have been reported here.
Mathys & Squire is delighted to announce that our trade mark team has been recommended in the 2024 edition of the World Trademark Review (WTR) 1000 guide. Partners Gary Johnston and Margaret Arnott retain their position as Recommended Individuals, whilst Rebecca Tew features for the first time.
The WTR 1000 directory illustrates the depth of expertise available to clients, serving as the definitive tool for those seeking outstanding trade mark services worldwide. Now in its 14th year, the WTR 1000 has firmly established itself as the definitive ‘go-to’ resource for those seeking stellar trademark expertise and partners worldwide. Mathys & Squire has been recommended for its work in the trade mark field, specifically in the ‘prosecution and strategy’ category. Our “team of experts share their clients’ ambition, passion and entrepreneurial spirit, going above and beyond what is expected when it comes to service delivery.”
Alongside our firm ranking, Partners Margaret (recommended in the categories of ‘Enforcement & Litigation’ and ‘Prosecution & Strategy’), Gary (recommended in the category of ‘Prosecution & Strategy’), and Rebecca (recommended in the category of ‘Prosecution & Strategy), have received the following testimonials:
“Margaret is a bright and responsive attorney whose team is very professional and extremely experienced, with expert knowledge of all trademark matters. They handle global trademark registrations with a deft touch and help to successfully resolve any international issues.”
“Gary is a well-respected IP practitioner who provides pragmatic and well-thought-out advice.”
“Rebecca is an impressive practitioner who successfully overcomes challenges and handles opposition proceedings through accurate strategic advice and strong arguments.”
We would like to thank each of our clients, contacts and peers who took the time to participate in the research. For more information and to see the full WTR 1000 rankings, please click here.