In this article for Business & Innovation Magazine, we summarise Mathys & Squire’s recent seminar entitled ‘Developing & implementing a commercially robust IP strategy’.
IP managers and decision makers for a number of varying sized companies attended, all of whom rely on innovation to set them apart from their competitors and ensure that they continue to prosper and grow.
We were delighted to welcome our guest speaker, Michael Edenborough QC – a seasoned silk and IP barrister who has been in practice for over 25 years. He was joined by Mathys & Squire partners Margaret Arnott and Dani Kramer, who shared their expertise in creating a formalised IP strategy; building a litigation strategy into business planning; and maximising the commercial value of IP.
Mr Edenborough kicked off the discussion, highlighting the importance of IP to business. He observed that IP is still undervalued by a majority of businesses, and that around 76% of companies do not have a formal IP strategy. As reported in Mathys & Squire’s recent publication ‘Trends in IP litigation’, around 77% of companies have not performed a formal IP audit and, as such, may not be cognisant of all of their IP. Registered rights, such as patents, registered designs and registered trade marks are reasonably easy to list. However, unregistered rights are often overlooked and, in many cases, are just as (if not more) valuable. These include know-how and trade secrets, which may be intangible but can be crucial to a business so it is important to recognise their full extent and include in your IP strategy a means for protecting/safeguarding them.
Mr Edenborough closed with his top tips for a robust commercial IP strategy in the form of ‘the five Es (see below).
Margaret Arnott followed with some advice relating specifically to trade marks, which provide the opportunity for strategic management. She recommended taking an ‘outside the box’ approach – in other words, don’t just consider ‘traditional’ marks, but think about whether there are other elements of your commercial ‘get up’ that could and should be protected to optimise your market position (e.g. the red soles of Christian Louboutin shoes).
Finally, Dani Kramer discussed registered designs, which protect the appearance of products. He explained that these rights are often under-utilised and overlooked, but that they can be a very powerful, (quick and relatively cheap), form of protection that can be enforced against early competitors and copycats. Registered design protection can be considered for more than just a product itself, but also for packaging, user interfaces, icons, logos and patterns/surface finish.
Whether or not a company deals in technical innovation, the message is clear: a robust IP strategy would include ensuring that all important elements of your products/services are covered by registered rights such as patents, registered designs, and registered trade marks, but also ensure that you know what unregistered IP rights you have, and how to protect/safeguard them. Once you have performed an IP audit, develop an IP strategy following the five Es, and final, remember that contentious issues rarely progress to full court proceedings: there are many different litigation strategies available that can be conducted and concluded at relatively little cost.
IP strategy – the basics
- Perform an IP audit (updated regularly) so that you know exactly what IP you have
- Use and protect what you now know you have or will have
- Leverage what you have to its fullest extent
- Consider the five ‘Es’:
Education: ensure you know what you have, how to identify elements of IP, and when protection is required.
Entitlement: who owns the IP? Contractors? Joint Venture? Collaboration Agreement?
Exploitation: are you going to manufacture? Or license? If licensing, what type of license will you offer?
Enforcement: do you have a strategy in place for identifying and quickly dealing with infringement of your rights?
Evasion: what do you do to ensure that you are not infringing others’ IP rights?
This article was first published in Business & Innovation Magazine in March 2020.
The British Government has announced that the UK will not be participating in the proposed Unitary Patent and Unified Patent Court (UPC). This puts an end to several years of speculation following the result of the ‘Brexit’ referendum in 2016.
This news was first shared by the UK’s BioIndustry Association following a verbal communication from the Intellectual Property Office, which had previously been working on the UK’s preparations for implementation of the Agreement establishing the Unified Patent Court and the Unitary Patent. It was then confirmed by a government spokesperson, according to reports in publications IAM and Managing IP.
The proposed Unitary Patent would have provided a single patent covering multiple countries, as an alternative to the current European system in which patent applications are validated on a country-by-country basis. The Unified Patent Court would have provided a single pan-European patent court system with divisions throughout Europe, with exclusive jurisdiction over validity and infringement of unitary patents and – eventually – exclusive jurisdiction over European patents granted for participating countries via the current system too. A specialist part of the court’s Central Division, responsible for chemical and biotechnological patents, was to have been located in London.
Although the UPC Agreement is not formally EU legislation, membership is only open to EU member states and the Agreement requires the court to apply EU law insofar as it relates to patents; this includes, for example, EU legislation relating to SPCs and biotechnological inventions. It also requires the UPC to refer questions to the European Court of Justice (CJEU) where questions about the interpretation of such legislation arise. Following the Brexit referendum, there was therefore doubt as to whether the UK would seek to continue to participate, and whether this was legally achievable.
Ultimately, it seems that the involvement of the CJEU, however indirect, was a sticking point for the UK Government in view of its stated desire to escape the CJEU’s jurisdiction entirely.
Following the UK’s announcement, the future of the whole project may be in doubt in view of the UK’s importance as a major economic centre with significant patenting activity, and a highly experienced and respected IP judiciary which will no longer form part of the UPC system. At the very least, a significant delay can now be expected while the consequences of this latest version of ‘Brexit’ are digested and consequential amendments to the UPC Agreement are considered. Another question mark is currently hovering over the future of the UPC due to a legal challenge in Germany which contests whether the UPC Agreement is compatible with the German constitution. A decision in that case is expected later this year.
Whatever happens to the UPC, this will not affect the current European patent system administered by the European Patent Office (EPO). This is not an EU institution and Brexit does not affect the ability of UK-based patent attorneys to secure and defend patents at the EPO.
For more information on Brexit and its effects on IP, click here.
Alex has also provided his comments on this news story for Kluwer Patent Blog – click here to read the full article.
As competition drives innovation and awakens the creative spirit, we discover that brand identity is, now more than ever, paramount to staying memorable.
It is therefore not surprising that businesses will extend their creative efforts far beyond choosing a brand name and logo. Indeed, elements such as colour schemes, packaging, product designs and brand ethos are becoming the centre of attention.
As such, we believe that it is essential for brands not only to recognise the strongest elements of their identity, but to also protect them accordingly.
This article will briefly touch upon a few key considerations for an all-inclusive brand protection strategy.
Name and logo
The process of registering names and logos is perhaps the most common and familiar to most businesses, usually representing the first step in protecting the brand. However, it is worth noting that certain logos (and names if stylised in an original way) may also give rise to copyright protection. This is particularly important where a third party may apply an identical/highly similar sign to dissimilar goods/services, as copyright protection is not limited by classes and can be enforced against trade mark applications/registrations across all industry sectors.
In the UK, copyright arises automatically, but it is essential for brand owners to keep records of the time of creation and ensure they legally own the copyright in their marks; this should be particularly considered where third party designers/agencies as well as company directors are engaged in producing the creative work.
In other countries, for example in the US or China, it is possible to officially register copyright, which again may prove highly useful in enforcement across all classes of goods and services. This can be notably beneficial in territories such as China, where businesses may fall victim to brand hijackers.
Protected Designations of Origin (PDOs) and Protected Geographical Indications (PGIs)
We have seen lately a growing interest among consumers in knowing where their products come from and how they were produced. Protected Designations of Origin (PDOs) and Protected Geographical Indications (PGIs) can be effectively used by brand owners to reassure the public and certify particular qualities of their goods.
These types of rights are especially relevant for the food and drink sector, as they guarantee the location where goods are grown and prepared, as well as the methods, processes (usually traditional) and standards of production.
Cheddar cheese, Cumberland sausages and Plymouth Gin are but a few examples of well-known PDOs/PGIs in the UK which consumers recognise and trust as having certain characteristics. Thus, if a business identifies added value in the place of manufacturing or methods of production, PDOs/PGIs are a route worth exploring. This approach can prove even more valuable when you consider that some geographical names may be difficult to protect as traditional trade marks, especially if there is an established connection between the place and the products in question.
Packaging and product design
In recent years, the presentation of a product has become equally important to its functionality. As such, businesses may find that consumers no longer choose primarily based on product performance, but focus instead on design and how it matches with their own style and values.
As increasing resources are being invested in developing attractive packaging and designs, it is essential to also understand how to best protect these valuable elements of a brand’s overall identity. There are multiple types of intellectual property rights associated with the appearance of a product; from copyright, to design rights and registered trade marks.
The original decoration of packaging may be protected by copyright or unregistered design rights, which arise automatically in the UK, but brand owners may also choose to protect such packaging by way of registered designs (e.g. the Pukka Tea graphic designs shown below which are registered at EU level).

A significant difference between copyright/unregistered design rights and registered design rights is that the former can only be enforced when designs are copied, whereas the latter also protects against independently conceived designs (which are deemed infringing).
In practice, this means that registered designs are much easier to enforce and due to the low fees associated with their registration, they may prove highly profitable in the long run.
Design protection also extends to 3D designs – brands such as Apple and Samsung, which focus greatly on the design of their products, also ensure their efforts are protected accordingly.
Businesses have also attempted to register the shapes of their products as trade marks – the reason being that registered designs are only valid for 25 years, whereas trade marks can remain valid for life so long as they are renewed.
As such, where brands have significant added value in the shape of their products, they may seek trade mark protection so as to ensure its monopoly is not limited in time. Examples include the classic 1980s Coca Cola bottle and the Toblerone chocolate bar.
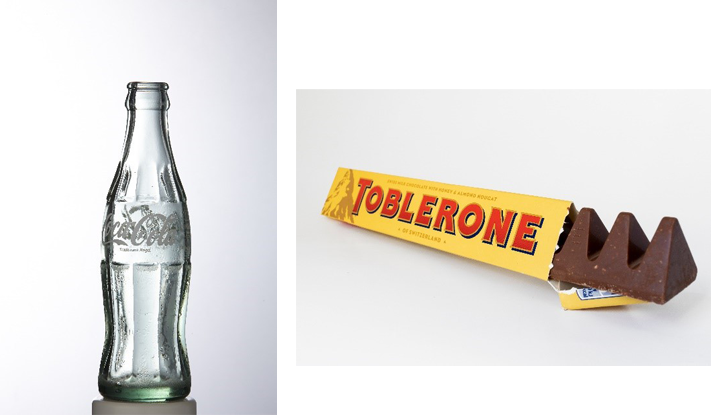
Colour scheme
Colours as such are likely to be deemed devoid of distinctive character and therefore not suitable for trade mark protection.
Nevertheless, businesses may notice that, following prolonged use of a colour in connection with their goods/services, consumers start associating it with their brand. Whilst this is a good thing, it can also mean that third parties may try to take unfair advantage of the consumers’ perception and cause confusion in the market.
In such scenarios, it is advisable to try and register the colour in question, which will, at that stage, have acquired distinctiveness following the use made of it. Famous examples of colour trade marks include Tiffany Blue and T-Mobile Magenta.


To sum up…
In conclusion, we have seen that there are various key elements in a brand which can bring added value to the business. As such, brand owners are advised to timely assess all these elements and identify not only how to profit from them now, but also how to adequately protect them and benefit from them in the long run.
Intellectual property law offers various ‘tools’ and ‘instruments’ that can be tailored, combined and adapted to a brand’s specific needs. These go far beyond the traditional trade mark registrations and should be carefully considered for an all-inclusive brand protection strategy.
Business owners are therefore advised to work together with their attorneys to put in place a bespoke strategy that best suits their individual needs and future plans for expansion.
In this article for Open Access Government, Mathys & Squire managing associate Charlie Dempster provides a focus on chemistry, specifically detailing nanocellulose in water purification.
Water purification technologies are becoming of increasing importance in modern society. Various innovative solutions are being developed by businesses to address the issue of water purification. Intellectual property such as patents can be used to help these organisations gain an upper hand over their competitors.
Water purification processes are essential for the provision of an adequate supply of drinking water for the world’s population. Water purification is also important in various industries such as chemical, pharmaceutical and wastewater management. It is estimated that of the millions of people that die around the world each year from infections such as diarrheal disease, a large number of these infections could have been prevented by access to safe drinking water(1).
Filtration technology
Filtration is a key technology used in water purification. In recent years, there has been an increasing interest in using nanomaterials in membranes for water filtration, which are considered attractive due to their larger surface area compared to bulk particles. The surfaces of many nanomaterials can also be modified by chemical treatment, enabling the nanomaterial to be tailored for removal of a particular contaminant. A nanomaterial is typically understood to be composed of particles that have at least one dimension of 1 nm-100 nm in size. Numerous types of nanomaterials have been studied for potential use in water purification processes, including nanocellulose, carbon nanotubes, graphene and its derivatives, and dendritic polymers(2).
Nanocellulose filtration
Of these materials, nanocellulose has attracted considerable attention since it is an abundant renewal material, derived from cellulose – the most abundant naturally occurring polymer on earth. It is produced by and can be extracted from a great many plants and is also chemically inert with good mechanical strength, meaning it is suitable for use in filtration membranes. Nanocellulose has an abundance of hydroxyl groups upon its surface. This property, along with its large surface area, enables nanocellulose to be chemically treated in a variety of different ways so as to have an affinity towards a particular contaminant or pollutant that it is desired to remove during water purification(2), (3), (4).
Examples of nanocellulose surface modification include carboxylation, sulfonation, phosphorylation and esterification of the nanocellulose surfaces. The surface modification is selected based upon the contaminant desired to be removed from the water. For example, negatively charged functional groups such as carboxylate and sulphate groups can be introduced if it is desired to remove positively charged contaminants from the water (such as various toxic metal ions). Similarly, positively charged functional groups can be introduced if it is desired to remove negatively charged contaminants. It has also been possible to remove organic pollutants such as dyes, pharmaceuticals, oils and pesticides from water with nanocellulose functionalised with hydrophobic groups that have an affinity for these molecules(3).
Nanocellulose exists as cellulose nanocrystal (CNC) or cellulose nanofibers (CNF). CNF is composed of cellulose fibrils that are typically from 2 nm – 20 nm in width, with a much longer length. CNC is composed of nanoparticles that are shorter in length than the CNF fibres(3). Preparation of nanocellulose filtration membranes typically involves extracting cellulose from plants before chemically treating the cellulose and then membrane formation. Conventional techniques for nanocellulose extraction involve using technologies known in the paper industry. However, there have been significant advances in the last decade in nanocellulose extraction: a key development was the use of TEMPO (2, 2, 6, 6-tetramethylpiperidine-1-oxyl radical)-mediated oxidation of wood cellulose. The method is described in Isogai et al(5) and involves TEMPO-mediated oxidation of wood cellulose in water to produce cellulose nanofibers containing C6 carboxylate groups. In this method, the negatively charged carboxylate groups formed by the oxidation electrostatically repel each other, causing the fibres to separate upon gentle mechanical disintegration. The method thus involves both extraction and surface modification of the nanocellulose.
Nitro-oxidation method
Methods such as those discussed above typically involve extracting and pre-treating the cellulose before the TEMPO-oxidation and subsequent mechanical homogenisation. A different and more recent approach is discussed in Sharma et al.(6) in which a nitro-oxidation method was developed to prepare carboxylated CNF directly from untreated plant material by treating the plant material with nitric acid or sodium nitrite. The method is believed to be a more economical process since it requires less processing steps(4).
For nanocellulose-based filtration technology to be commercially implemented on a large scale, cost-efficient processing routes of surface modified nanocellulose must continue to be developed. It will also be necessary to continue to investigate the selectivity of nanocellulose-based membranes for a variety of different pollutants and contaminants, which will likely require further development of the surface modification technologies discussed above(3).
For enterprises involved in commercialising nanocellulose-based membrane technology, protecting their innovations in this rapidly developing field will be vital for gaining a competitive advantage. Patents enable businesses to prevent competitors from using the patented technologies in the jurisdictions in which they are in force and can also be used to generate revenue by licencing patented technology to third parties. Patents could be directed to novel processes for the extraction of nanocellulose from plants, synthetic routes to surface modify the nanocellulose, or new methods of membrane fabrication. Similarly, patents can protect new forms of surface modified nanocellulose, or new filtration membrane structures (e.g. hybrid membranes containing nanocellulose and other materials).
This article was originally published in Open Access Government in February 2020.
References
(1) Combating Waterborne Diseases at the Household Level, World Health Organization. 2007. Part 1. ISBN 978-92-4-159522-3
(2) Nanoscale Materials in Water Purification, Thomas et al., Elsevier, 2019
(3) Nanocellulose-based materials for water purification, Voisin et al., Nanomaterials, 2017, 7, 57
(4) Chemistry: Sustainable water purification solutions from underutilised biomass, https://www.openaccessgovernment.org/sustainable-water-purification/74400/
(5) TEMPO-oxidized cellulose nanofibers, Isogai et al., Nanoscale 2011, 3, 71 to 85
(6) A simple approach to prepare carboxycellulose nanofibers from untreated biomass, Sharma et al., Biomacromolecules, 18 (8), 2333-2342, 2017
The high-profile case of Shnuggle v Munchkin has a significant impact on the way designs are registered. In this article for Intellectual Property Magazine (an extended edition of his original comments in November 2019), associate Max Thoma analyses the implications.
Shnuggle v Munchkin shows the continuing impact of the Magmatic Ltd v PMS Intl Ltd (Trunki) Supreme Court decision of 2016.
This Intellectual Property Enterprise Court (IPEC) case related to a dispute about design rights in baby baths. Shnuggle, a Northern Irish manufacturing company, alleged that Munchkin, a large US-based competitor (and the co-defendant Lindam, a subsidiary of Munchkin), infringed Shnuggle’s two registered Community designs and their UK unregistered design rights, related to its eponymous Shnuggle baby bath by producing and selling a product named Sit & Soak.
Shnuggle’s earliest registered design seems to have been the output of a Computer-aided design (CAD) package, which commonly show drawings in a solid colour (blue in this case) so as to indicate curvature to the user of the
CAD package. Shnuggle opted to file the ‘raw’ blue drawings in its registered design application, rather than converting them to line drawings. This resulted in Her Honour Judge Melissa Clarke interpreting the drawings as being limited to the colour blue, following the Trunki decision, as opposed to the white colour of the allegedly infringing product. This emphasises the dangers inherent in the use of easily accessible drawings in registered design applications, rather than drawings which are properly prepared.
However, in this case, the judge made the point that limitation to the blue colour was not determinative in the outcome of the case. The Sit & Soak was found not to infringe Shnuggle’s first registered design as a result of the significantly different overall impression created by the elongated ‘teardrop’ shape of the back of the Sit & Soak and the ‘floating edge’ on the front and side of the Sit & Soak (among other less important differences, including the white colour).
Shnuggle’s second registered design was based on line drawings and was seemingly marginally more similar to the Sit & Soak. However, the judge considered that it did not produce a different overall impression to Shnuggle’s first registered design, and so the second registered design was invalid in the light of the first. For good measure, the judge also commented that even if the second registered design were valid, the Sit & Soak would not infringe for the same reasons given in relation to the first registered design. Thus, although it is probable that Shnuggle would have been in a somewhat better position if it had filed a more considered initial design application based on line drawings, the decision seems to indicate that the judge would have in any case considered the differences to be too great for a registered design infringement claim to succeed.
With its registered design infringement claims failing, Shnuggle was forced to rely on its claims for infringement of design right, which were based on various sub-sections of the Shnuggle product, rather than the whole product. There was some discussion whether these sub-sections were “part of an article” following the amendment to the relevant law by the Intellectual Property Act 2014. In particular, Munchkin submitted that a “part” must be separately created, rather than being a disembodied part of a single article. The judge disagreed with this particular contention, but reaffirmed other judges’ views in recent decisions that the part cannot be disembodied and abstract. The judge summarised that a part of an article is “…an actual, but not abstract part which can be identified as such and which is not a trivial feature.” This provides useful guidance for designers and manufacturers as to what can be asserted in a design right claim.
Although Shnuggle succeeded in showing that its claim related to actual “parts”, many of these parts, relating to the design of the most recent Shnuggle product, were deemed not to be original over a previous version of the product, or otherwise simply commonplace. The design right in the exterior of the sides and base of the original Shnuggle was considered to be original and to have been copied by Munchkin from the Shnuggle product – but since this copying was not exact or substantial, the design right claim failed.
This case shows the pitfalls inherent in attempting to enforce unregistered design rights in the absence of a suitable registered design. The case also reiterates the importance of filing the correct drawings in a registered design application. It may be easy to feel sympathy for Shnuggle, who effectively failed at the final hurdle to show infringement of their design right – and it will certainly be interesting to see if an appeal results.
This article was originally published in Intellectual Property Magazine in February 2020.
From big data to database rights, in this article for Open Access Government, Mathys & Squire partner Sean Leach explains the role technology plays in the future of healthcare.
Technological developments in the collection and usage of clinical data create new opportunities for improving patient care and identifying treatments.
To take full commercial advantage of these developments and keep the edge gained by innovation, intellectual property (IP) and confidential information must be safeguarded.
The strategy for doing so cannot follow the legacy model used in neighbouring fields such as medical devices or pharmaceuticals. A new approach to IP must take into account the technical and commercial reality of these new technologies.
Big data
Data collection in healthcare is changing, both in terms of the volume of data that is collected and the level of clinical detail it describes. Clinicians may now record data at the bedside in electronic patient records, and so-called point of care diagnostic testing devices provide a further data stream. Patient records may also include information about drug treatments, health history, and traditional diagnostic information such as radiography, biopsies and so forth. The sheer volume of data available, even about one individual patient, is enormous. Indeed, there is so much data that it can in some circumstances exceed a clinician’s ability to assimilate and use it all.
Big data and machine learning techniques offer exciting possibilities to filter mass data or draw insights from it to support clinical decision making. Data mining also offers a way to uncover new treatments and to
change or improve existing treatments, for example, in how drugs are delivered. This might mean adopting a different dosage regimen for different cohorts of patients.
There is huge commercial potential in these techniques, so there is a need to license them in order to promote their use and to protect the IP.
A challenging IP landscape
Database rights
Traditional protections for IP in healthcare may not work for big data innovation, and recent changes create issues for the licensing of these technologies.
In the same way that source code is key in software innovation, the training data upon which predictive models are based can also be fundamental to the development of machine learning techniques. Databases of such data, therefore, have significant value in their own right.
One of the most relevant types of IP protection is the sui generis database rights that were introduced by the Database Directive. These provide database owners with the right to prevent the unauthorised copying or extraction of data from their databases in the European Economic Area (EEA). After the end of the Brexit transition period (i.e. as of 1 January 2021), UK citizens, residents and businesses will no longer be eligible to receive or hold sui generis database rights in the EEA. However, database rights that exist in the UK or EEA before the end of the transition period (whether held by UK or EEA persons or businesses) will continue to exist in the UK and EEA for the rest of their duration.
Any IP licence which includes the licensing of database rights must take account of this change. One option, if circumstances permit, is to use neighbouring rights such as copyright and rights in confidential information. Proper drafting of the relevant licence and control of the information exchanged under that licence, may be vital if control of this valuable IP is not to be lost.
Patents
Obtaining patents for software can be difficult. Happily, in the field of healthcare innovation, this can be easier than in other technologies. So, patent protection for software in this area should not be ruled out.
Where machine learning techniques are involved, the difficulty of defining how the underlying technique actually solves a particular problem adds a further complication. It might be the case that the innovation lies in the manner in which training data is pre-conditioned, rather than in the design of the algorithm itself. In addition, machine learning innovation is often implemented ‘in the cloud’, and the processing engine itself may never be distributed. In so far as the customer is concerned, the technology is just a ‘black-box’. This creates a difficulty in policing patent infringement, which must be weighed against the need to disclose the details of an invention in any patent. This is a real consideration, and patents must be drafted carefully with this in mind.
This does not mean that patents are irrelevant in this space. At the very least, there is a risk that an infringement believed to be hidden might be discovered, and the financial and reputational damage that would arise
cannot be dismissed. In practice, these decisions are made by individuals – CEOs and General Counsel – who are then accountable to their board/shareholders for that decision. In that context, legal advice which says infringement will not be detectable is not to be given or accepted lightly. In addition, there is the question of what would be done in the event that an invention is kept secret but subsequently patented by a competitor. The original inventor would then be left to decide whether they were prepared to run that risk themselves and rely only on the very narrow defence provided by their own secret prior use. The right decision may well be not to file a patent application, but that decision should be taken positively, with full awareness of the costs and benefits.
Final thoughts
Big data and machine learning techniques in general and their application to healthcare in particular, are generating exciting new opportunities. The circumstances of each case are unique, and raise complex new issues as the regulatory, legal and technical landscape evolves. Seizing those opportunities requires an IP strategy which is adapted for those circumstances and is far-sighted enough to see the next challenge coming.
This article was originally published in Open Access Government in February 2020.
Mathys & Squire is delighted to be recommended in the 2020 edition of the World Trademark Review (WTR) 1000 directory. Trade mark partners Margaret Arnott and Gary Johnston have also maintained their statuses as recommended individuals.
The WTR 1000 recommends leading national and international trade mark practitioners, illustrating the depth of expertise available to brand owners as they seek to protect their brands. The guide serves as the definitive tool to locating the best trade mark partners worldwide.
Praise for Mathys & Squire in the 2020 edition includes: “Mathys’ advice is plotted on a spectrum of risk to cost and is always formulated with sensitivity to the wider commercial and industry context.”
Individually, the co-heads of our trade mark practice have been ranked in the categories of ‘Enforcement and litigation’ (Margaret Arnott) and ‘Prosecution and strategy’ (Margaret Arnott and Gary Johnston):
‘London-based Margaret Arnott heads up the litigation team. “Her incisive and pragmatic approach, combined with her strong grasp of current market trends, saves clients time and money. Reasonable and level-headed, Margaret is adept at curtailing situations in which adrenaline and emotions are running high.”
“She is a privilege to work with. A true accelerator of businesses, she is knowledgeable, approachable and proactive.”‘
‘UK and European trademark and design attorney Gary Johnston “sees the big picture and is able to plan and execute sensibly and reliably”. Based in Manchester, he leverages his 25 years’ experience in anti-counterfeiting, global portfolio management and filing to his patrons’ best advantage.’
For more information and to see the full WTR 1000 rankings, please click here.

In the second of a series of articles for IBI Journal, Mathys & Squire partner Anna Gregson, who specialises in biotechnology, provides an overview of some of the key applications of cell therapies as well as a closer look at the challenges facing the evolution of this field.
The achievements of cell-based therapeutics over the last decades have bolstered efforts in recent years to bring more of these products to market and across an ever more diverse range of applications. These advanced therapeutics offer promising potential to treat conditions which, to date, have defied traditional treatment modalities. Interest and investment in this sector is at an all-time high and whilst many are hopeful of a boom in the number of approved therapies in the coming years, the industry still faces significant challenges, particularly with regard to the manufacture and regulation of these cell-based products.
To date, the applications of cell therapies have largely fallen into two broad categories; tissue regeneration and immuno-modulation. With regard to the former, cell therapy has been viewed as one of the most promising techniques for the repair of damaged tissue, with applications in cardiovascular disease, neurodegenerative disease (for example, Parkinson’s and Alzheimer’s), musculoskeletal injury or degeneration and endocrine dysfunction (for example, type I diabetes).
Cell therapies have proven particularly effective in the repair of articular cartilage, for which the intrinsic capacity for repair is low. The most established of these therapies have employed the patient’s own cells, i.e. autologous cells. In brief, harvested chondrocytes are expanded ex vivo, seeded into a collagen matrix and then re-implanted into cartilage defects in joints. Such products have been available for around a decade now (ChondroCelect, developed by TiGenix was first approved in the EU in 2009) and have shown considerable efficacy, although use of these advanced options is still low when compared to traditional treatment modalities (for example, joint replacement and analgesics). Whilst cartilage repair applications have tended to employ the terminally differentiated chondrocyte, bone repair applications have made use of the regenerative capacity of stem and progenitor cells. Bone marrow derived mesenchymal stem cells (MSCs) have been proven in a range of orthopaedic applications over recent decades, including in the treatment of infants with osteogenesis imperfecta and in the repair of non-union fractures. Unfortunately, obtaining sufficient yields of pure MSC populations from bone marrow has proven difficult and there has been a switch in recent years to utilise MSCs derived from other sources, such as adipose tissue.
Autologous cell therapies like those discussed above all depend on obtaining sufficient cell numbers from the donor patient and the ability to expand functional cells ex vivo. Off-the-shelf cell therapies, which clinicians can employ for a range of patients, as and when needed, without concerns over yield or expansion protocols, are likely to represent the future of cell therapy. UK based biotech, ReNeuron is one such company forging ahead with allogeneic cell therapies. Interestingly, ReNeuron’s neural stem cell line for the treatment of the disabling effects of stroke were cryopreserved prior to utilisation in the PISCES I (phase I) clinical trial. Cryopreservation is just one of a number of advancements which will be necessary to bring off-the-shelf cell products to reality.
Whilst the regenerative applications of cell therapies have, at the very least, been researched for some time now, the immuno-modulatory applications of cell therapy, in particular, chimeric antigen receptor (CAR) T cells, is a more recent development. Indeed, it was only in the early 90s when first generation CAR T cells (which contained an antibody/T cell receptor fusion molecule) were developed and around the same time researchers were investigating adoptive transfer of patient derived virus-specific T cells. Since these early days, significant leaps forward have been made. In 2017, Novartis’ Kymriah (tisagenlecleucel) became the first CAR T cell therapy to be approved by the FDA, with Kite Pharma’s Yescarta (axicabtagene ciloleucel) following shortly thereafter. Data from the UK’s Cell and Gene Therapy Catapult clinical trials database indicates that there were around 22 clinical trials investigating the safety and efficacy of CAR T cells in the UK alone in 2018. The success of CAR T cells to date has largely been shown for haematological malignancies (indeed, Kymriah and Yescarta are approved for the treatment of acute lymphoblastic leukaemia and large B-cell lymphoma respectively). In contrast, despite extensive research, CAR T cell therapy for solid tumours hasn’t had the same impact, not least because of the challenges of targeting solid tumours including identifying a suitable target antigen and homing the cells to the hostile, tumour microenvironment. Nonetheless, strides are being made by combining CAR T cell therapy with other biologic agents, specifically checkpoint inhibitors such as pembrolizumab and nivolumab which target programmed cell death protein 1 (PD-1) a key regulatory protein found on T cells. The University of Pennsylvania, for example, is recruiting for a phase I clinical trial assessing the safety of a CAR T cell/pembrolizumab combination therapy for the treatment of glioblastoma. This follows preliminary evidence from the Memorial Sloan Kettering Cancer Center that showed both safety and efficacy of a mesothelin targeting CAR T cell and pembrolizumab combination therapy in patients with malignant pleural disease. Thus, the use of CAR T cells for the treatment of solid tumours appears to be progressing.
Immuno-modulatory cell therapies other than CAR T cells are also being investigated in the clinics. By way of example, Fate Therapeutics is currently assessing the safety of its off-the-shelf Natural Killer (NK) cell therapy. Unlike traditional CAR T cells, Fate’s NK cell therapies are derived from an induced pluripotent stem cell (iPSC) line allowing the production of large numbers of well-defined cells without relying on a patient’s own immune cells (which are often depleted in many cancers). Preclinical studies showed the efficacy of these cells in the treatment of checkpoint inhibitor resistance tumours. At present, Fate has a pipeline of at least five different NK cell therapies.
As well as the immuno-oncology applications, cell therapies are also being trialled for immuno-regulatory applications such as for in the treatment of autoimmune disease and graft versus host disease. These trials have largely involved the use of autologous, expanded, regulatory T cells (Treg cells) which, through a range of mechanisms, are able suppress a variety of immune cells. Treg cells used in studies to date have been isolated from both umbilical-cord blood and peripheral blood. A variety of phase I studies have been completed or are in the process of assessing the safety of Treg cells for the treatment of type I diabetes. Although in the early stages of development, data to date is showing that Treg cells are well tolerated in patients and the ex vivo expansion methods are capable of generating sufficient numbers of stable and functional Treg cells. Future phase II/III trials will of course be needed to reveal the true potential of these cells.
Global investment in cell-based therapies increased to US$7.6 billion in 2018, a 64% increase from the previous year. In spite of this, the sector still faces a number of significant challenges before these advanced therapeutics become widely used.
Research and development in this sector is undeniably booming, though difficulties in expanding, manufacturing and transporting cell products may be hampering the commercial viability and ultimate availability of these products. Achieving the quantity of cells needed with current production methods, especially if uptake of these therapies becomes more widespread, is one of the major hurdles facing the industry. By way of example, the recommended dose of ChondroCelect is 1 million cells/cm2 of cartilage defect. CAR T cell therapy Yescarta is dosed at a staggering 2 million cells per kg (around 140 million cells for an average adult male). The issue is magnified somewhat by the focus of today’s research on the cell product per se; emerging biotech companies with innovative cell therapies should, at an early stage, consider the processes that will be necessary to achieve the desired cell numbers for later phase II/III trials and beyond. These challenges also bring opportunities however, and there are now a number of innovative companies seeking to develop solutions for the industry, to simplify, accelerate and improve cell therapy manufacturing and supply.
Automation of the manufacturing processes is currently of significant interest to the community. At present, the manufacturing processes employed in the generation of cell therapies largely resemble those utilised in other biopharmaceutical areas (for example therapeutic antibodies). Unlike therapeutic antibodies production however, cell therapies (especially those relying on patient or donor cells) vary significantly from batch to batch, requiring complex and adaptive processes to generate consistent products within the regulatory confines. Through the implementation and training of a variety of mechanisms, e.g. sensors, robotics and image acquisition as well as processing software, researchers believe variability and reliability of current manufacturing processes can be improved.
Whilst improvements in the manufacturing processes will hopefully lead to a reduction in the costs associated with the production of cell therapies, it should be noted that, unlike traditional therapeutic modalities, cell therapies are often a one-off treatment option for patients. Biotech companies must bear this in mind when attempting to recoup their research and development costs and, as such, costs are always likely to be higher than traditional biologics. As it stands, the high costs associated with these therapies is proving challenging for healthcare providers to justify.
The cost of these therapies is at least in part due to the convoluted path from bench to bedside. Cell therapies are considered differently to the conventional biopharmaceutical agents and have to undergo even more rigorous regulatory and quality assessments. This of course ensures public safety, but has also put the brakes on the number of cell therapies actually being approved (despite the ample number of trials). As is so often the case, the regulatory frameworks in place have not been able to keep up with the unprecedented scientific advances in this field. What’s more, the absence of harmonisation across jurisdictions has placed undue burden on the smaller players in this field. The lengthy timescales involved in obtaining regulatory approval (even after showing clinical efficacy) are exemplified by Holclar, an autologous cell therapy (comprising human corneal epithelial cells and limbal stem cells) for the repair of damaged cornea, which despite having shown clinical efficacy as early as 1997, only obtained regulatory approval in 2015.
Regulation is of course paramount to ensure the safety of patients receiving advanced therapeutics (including cell and gene therapies) which have long been shrouded in safety concerns. These concerns are not without basis. Indeed, safety has been a major sticking point for stem cell therapies. The primary concern regarding stem cell therapies is unwanted differentiation, as has been shown in the cardiovascular setting, where calcifications have been identified in the myocardium of patients treated with MSCs following infarction (MSCs, of course, give rise to cells of bone and cartilage as well as muscle). Tumorigenesis has also been a concern for stem cell therapies, although this appears to have been unwarranted based on current data. In the immuno-oncology field, CAR T cells have also been associated with safety concerns including the development of cytokine release syndrome in patients receiving CAR T cell therapies, the engagement of target antigens on non-pathogenic tissues and host immune response to the specific recombinant proteins found in these cells. Pleasingly, the industry is seeking solutions to these problems and research is ongoing to improve the safety profile of these therapies. In the CAR T cell space, the incorporation of suicide or elimination genes into delivered cells is being investigated as a means to selectively deplete these cells in the body when necessary. The approved cell therapies are largely still in their infancy and data from future phase IV clinical trials will be indispensable in assessing the long-term safety of these therapies.
The number of cell therapies actually approved for clinical use remains small. This highlights that, despite the significant scientific advances and investment, the sector is largely still at the research and development stage. Having said that, the industry appears to have reached a critical mass and with the number of clinical trials in this field growing steadily, we can only assume that we will be seeing more and more of these therapies in the clinics. The industry seems to have clicked and more emphasis is now being placed on the challenges of efficiently, yet safely, manufacturing these products. Improvements in this key area could pave the way for wider implementation and access to these therapies. A multidisciplinary approach will be essential in the coming years to increase the number of approved therapies whilst still ensuring affordability and, importantly, patient safety.
This article was first published in the Winter 2019 edition of IBI Journal (pp. 6-9).
In this article for Food Manufacture, managing associate Laura Clews considers some examples of innovation in alternatives to single-use plastics.
For decades, many countries around the world simply exported recyclable materials, including plastics, to China for reuse in their recycling programs; in fact almost half of the world’s recycling was exported to China as this was more economical than developing national recycling programs. However, in 2018, China announced a ban on importing waste, including plastics, citing environmental reasons for this change. Accordingly, many countries around the world must either develop economical recycling facilities or turn to the use of landfill sites (which could not be used indefinitely!).
Through the actions of many people in the media spotlight, such as David Attenborough and Greta Thunberg, the environmental impact of day-to-day life has also been brought to the forefront of consumers’ concerns. In particular, consumers are looking to more environmentally friendly alternatives to single use plastics within the food and drink industry.
Thus, endeavouring to meet these needs, many companies within this industry have funded intensive research to find environmentally friendly and economically viable methods of recycling plastic and/or suitable alternative packaging materials.
Recycling plastics
One company looking to find more effective and environmentally friendly methods of recycling plastics is Green Lizard Technologies. The method of recycling waste polyethylene terephthalate (PET) developed by this company uses proprietary catalyst systems to break down the PET polymer chain to its raw materials (BHET – bis(hydroxyethyl)terephthalate). It has been reported that this new method is highly effective, producing yields of BHET of up to 61% from the reaction mixture. Once PET has been broken down to BHET it can then be reused to form other plastics or alternative polymeric materials.
It has been reported that this process produces recycled materials which are essentially free from contaminants, and therefore can be reused to produce food and drink packaging, such as PET recycled water bottles.
This new recycling method has caught the eye of major players within the industry, and it was announced on 14 November 2019 that Poseidon Plastics Ltd., a joint venture between Green Lizard Technologies, Panima Capital & Abundia Industries, signed an agreement with the world’s leading differentiated producer of PET and PEN polyester films, DuPoint Teijin Films (DTF) in order to develop this unique polyester recycling technology.
Is replacing single use plastics with alternative materials really the answer?
A report by Green Alliance published in January 2020, ‘Plastic promises: What the grocery sector is really doing about packaging’, looks at the kneejerk reaction by many retailers and suppliers to replace single use plastics with other materials which are considered to be “more environmentally friendly” in response to the increased media attention and growing pressures from the public.
The report comments that many retailers and suppliers are simply replacing single use plastic packaging with alternative single use materials, such as glass, paper, wood and biodegradable materials, without undertaking a rigorous analysis of the environmental impact of such materials compared to single use plastics.
Worryingly, many reports have found that due to the manufacturing process, number of times consumers are likely to reuse these materials and the availability of recycling schemes, these alternative materials can be more damaging to the environment than single use plastics. For example, a study for the Northern Ireland Assembly in 2011 found that paper bags generally require four times more energy to manufacture compared to plastic bags.
Alternative materials
Accordingly, there is a drive within the food and drink industry to source alternative materials which are more environmentally friendly and also meet the common requirements of the food and drink industry (such as preserving the packaged consumable).
For most adults (and particularly those who work in big cities and/or have children) coffee not simply a drink choice, but an essential for day-to-day life. In fact, it has been reported that in the UK 95 million cups of coffee are consumed a day, which produces around 500,000 tonnes of used coffee grounds every year, most of which simply ends up on landfill sites (see ‘Put Your Coffee Waste to Work’, by Bio-bean). Fortunately, Berlin based company, Kaffeeform, has found a way to repurpose this waste product, turning coffee grounds into durable cups. Kaffeeform states that these alternative cups are formed from recycled coffee grounds and other plant-based resources that are hardened with biopolymers.
In addition, New York based biotech company, Ecovative Design, has also produced a new environmentally friendly material based on mushrooms. The material has been used to form surfboard blanks to replace those previously used formed from expanded polystyrene or polyurethane foam with a fibreglass coating.
The blanks, which are entirely biodegradable, are made from a material called Myco foam which is formed from mycelium (the white, glue-like, branching part of fungus referred to as hyphae) and organic farm waste, such as corn husks, straw and lentil pods. During manufacture the mycelium grows, feeding on the organic waste, and forms long entangled fibres. This new material has been an immediate success, and not only in the surfing world. Ecovatve Design has now branched out into making faux-leather materials, packaging and skincare products, forming partnerships with well-known companies such as Bolt Threads, IKEA and DELL.
This article was first published in Food Manufacture Magazine in February 2020.
Today, 31 January 2020, the UK exits the European Union (EU). This however has no impact in practical terms on European IP rights for the time being, because after today the UK enters a transition period which is scheduled to last until 31 December 2020.
During the transition period, EU trade mark and registered design filings will continue to cover the UK.
European patents are in any case unaffected by Brexit (even after the transition period).
Mathys & Squire’s ability to represent its clients in the UK and Europe is not affected by Brexit (even after 31 December 2020). As a firm, we remain a European business with offices in the UK, Germany, Luxembourg and France, and we will continue to represent our clients across Europe.
Visit our Brexit page for more details on the long-term implications of Brexit on IP rights.
As a European firm, Mathys & Squire, with offices in the UK and Europe, we are in a strong position to be able to handle both UK trade marks and designs, as well as EU Trade Mark Registrations and Registered Community Designs, and we have been working with our clients on cost-effective strategies for doing so.
If you have any questions, please contact your usual Mathys & Squire adviser or for more information on this topic, email [email protected].