One of the most common issues faced when securing protection in China is the earlier registration of third-party rights, which are then cited against the later application. However, there are various tactics that can be adopted to overcome this hurdle.
Many articles are written about various aspects of the trade mark system in China: the risks of the first-to-file system, the development of the law in recent years, the behaviour of trade mark squatters and the production of counterfeit goods. However, fewer focus on the obstacles encountered when filing in China or offer practical guidance on how to overcome or address these.
For foreign businesses attempting to protect their marks in China, objections throughout the prosecution process are reasonably commonplace for various reasons, including significant differences between China’s legal system and those of other countries. However, in our experience, one of the most common issues is the earlier registration of third-party rights that are then cited against the later application and form a block to its progress. Under the Chinese system, the onus is on the applicant to overcome the possibility of confusion with earlier rights, as compared to it being up to the earlier rights holder to actively object to a later application.
Having assisted many clients over the years that face this exact problem, we have had to consider and develop
several potential strategies and solutions for tackling the issue. Below are a handful of the methods we regularly
use to try to obtain protection.
Pre-filing
Anyone filing in China should be aware that the classification system is unique and that a specification drafted for elsewhere in the world is unlikely to pass the Chinese examination process without some form
of query or objection being raised. Where possible, it is therefore advisable to obtain a full list of the terms included within each relevant sub-class from a Chinese attorney and to consider creating a separate or adapted specification for use in China that either utilises those terms in place of the specification employed in the jurisdiction where the mark was first registered or lists them alongside, to assist with later adaptations.
This will not always be possible (e.g. if filing via the Madrid Protocol or where a priority claim means that a previously drafted specification must be used). However, in instances where the strategy may be employed, it is advisable to consider doing so.
It will be useful from a wider prosecution perspective but can also help to avoid the unnecessary citation of earlier rights. For example, it can often be the inclusion of one term in one sub-class that prompts a citation from the China Trademark Office (CTMO). When, for example, a UK specification has been used, this may be an unnecessarily wide-reaching term that could have been removed or limited prior to examination in order to avoid the citation.
Conduct Searches as Soon as Possible
Pre-empt Objections
Despite the propaganda, the trade mark system in China no longer lives up to its Wild West reputation; there is a wider acknowledgement of the value of intellectual property and the authorities are adapting the law in an attempt to stamp out the dishonest practices that gave rise to the reputation in the first place. However, trade mark squatting is still present and there will always be individuals and businesses that see the financial opportunity presented by the first-tofile system.
For this reason, you will often find that a client’s brand is illegitimately registered in China even before that client has decided to protect the mark itself.
Because of this, as well as the potential existence of legitimately registered earlier rights, it is strongly advisable to conduct searches as soon as there is any indication that the client has an interest in the Chinese
market. Where no obstacles are found, early filing is also strongly recommended to prevent interim disruptive filings by third parties.
Searches and Non-use Actions
One of the most common methods of overcoming the citation of an earlier right is to file a cancellation action against the earlier right on the basis of non-use (i.e. where it has been registered for more than three years and no obvious use can be identified). This is a useful way of removing the citation from the register and thus clearing the way for the client’s application.
However, the process is not always straightforward; if the non-use action has been prompted by an official objection to a trade mark application, the CTMO will not generally agree to suspend examination of the application pending the outcome of the non-use action, thereby leaving the client with no option but to file the non-use action, abandon the application and re-file in the hope that by the time the new application is examined, the non-use action is close to being concluded.
This is why pre-filing searches are so important in China; it is much more beneficial to know about potential citations in advance of filing so that you can instigate those actions early, rather than wait 18 months until you receive a refusal and then potentially have to start from scratch. Indeed, in circumstances where nonuse actions are instigated before either the filing or the provisional refusal is issued, the CTMO is more likely to agree to a suspension pending the outcome of the cancellation action.
Thus, discovering potential citations before filing could lead to the development of a more informed strategy that reduces the time and cost involved in obtaining protection.
Invalidate Earlier Registrations
Where a non-use action is not possible (either because the registration is too young or use is being made in China), clients can also look at pursuing an invalidation action against the cited right.
These can be based upon a number of grounds, including earlier proprietary rights or bad faith; the latter of which is more likely to succeed when there is evidence of squatting and dishonest intentions.
However, such actions are generally difficult to support in the absence of good evidence, a copyright registration or prior use/protection of the mark in relation to virtually identical goods to those included within the cited registration.
Indeed, a handful of our clients have discovered identical logo marks registered in China in classes in which they have no protection and have not previously used their marks. In these circumstances, invalidation
actions failed despite the logo being clearly identical due to the lack of pertinent evidence of use or ownership in relation to the contentious goods or services.
Wait
Sometimes, despite all one’s best efforts, none of these options are viable and it is difficult to identify a route forward. In these circumstances, the client may need to accept that it has to play the long game. Generally if the application was filed by a squatter, there will be no real intention to use it and so you could choose to diarise the three-year non-use date and then pursue non-use proceedings as soon as possible,
while simultaneously re-filing for the client’s mark in the hope of achieving the above-mentioned stay of proceedings, if necessary.
Consider Copyright Protection
In the interim, or even as a defensive mechanism alongside an initial trade mark filing, copyright registration in China can provide a useful addition to a client’s portfolio and additional armour against trade mark
misuse or misappropriation. Indeed, it provides the client with a registered right protecting its stylised mark and it is not limited by the same classification system.
For clients with distinctive logos, a copyright registration can provide a strong alternative right for enforcement purposes. For example, the client can obtain a registered right to record with Customs, and potentially a broader-reaching right to enforce against squatters filing to protect the client’s logo in different classes of goods or services. It is therefore well worth considering.
Purchase Earlier Rights
It is no secret that trade mark squatters will often request huge sums of money in return for the transfer of an earlier trade mark registration for the client’s brand (or something similar). Often the figure put forward is far in excess of the value of that registration, particularly in view of the fact that squatters rarely take the steps to enforce those rights against the rightful owner.
However, in certain circumstances, the purchase of an earlier right may be worth considering – for example, if the value is reasonable in comparison to the cost of pursuing actions at the CTMO, potentially having to re-file and being forced to stall enforcement action in the interim.
Moreover, this could be an option worth considering where a mark is already within the non-use period or very close to that period and thus more vulnerable, or where there was actually a genuine intent to use but it has fallen out of use, or perhaps if there are overseas rights that can be used as leverage in negotiating a commercial relationship that could benefit both parties.
However, do consider who is best to begin those negotiations, as an initial approach from a lawyer is not always looked upon favourably from a cultural perspective.
This article was first published in the June/July 2019 edition of World Trademark Review.
For more information, contact Margaret Arnott or Laura West.
The food and drink industry is one of Europe’s largest manufacturing sectors, with, according to FoodDrinkEurope, an annual turnover of €1,109bn. However, in order to capitalise on this lucrative industry, manufacturers must follow (or more ideally stimulate) changing consumer demands and government initiatives.
One of the most significant recent consumer trends has been the increased demand for healthier foods and drinks. Consumers have become much more conscious of maintaining a healthy and balanced diet and are looking more critically at the ingredients contained within their food and drink.
In recent years there has also been an increased awareness of the methods of manufacture, the inclusion of additives and the sourcing of food and drink ingredients. The desire to live healthier lifestyles has resulted in, among others, an increased demand for new vegetarian and vegan options, nutritionally improved and ‘gut healthy’ foods.
This change in consumer purchasing is reflected in the given drivers for innovation in the food and drink industry, with health food trends (natural, medical and vegetal) reported as showing the greatest increase in 2017, particularly in the soft drink sector, which was recorded as the most innovative sector in 2017.
No doubt this change in manufacturing drivers is, at least partly, related to the new national guidelines, such as those published by Public Health England (PHE), which aim to reduce the amount of sugar in children’s food by 20% by the year 2020, and the general EU benchmark of reducing added sugars in food products by a minimum of 10% by 2020, with respect to the baseline levels of member states at the end of 2015.
But what if consumers grow tired of this healthy lifestyle trend and want to give in to ‘naughty’ urges once again? Is it possible to maintain a healthier lifestyle and continue to eat the comfort foods that we all know and love? Essentially, is it possible for us to have our cake and (quite literally) eat it too?
Fortunately, many food and drink manufacturers have committed extensive time and resources to looking at this exact issue. In recent years, research and innovation in the food and drink industry has not been solely focused on providing new healthy, micronutrient filled foods for us to try. It has also looked at how to make the food and drink products traditionally associated with higher calories and increased amounts of fat, salt and sugar healthier, while maintaining the texture, taste and appearance we have come to expect and enjoy.
Reduced Fat Fish and Chips
Fish and chips is quintessentially British fare, using a batter formed from a slurry of wheat flour and water. However, deep-fried foods
such as these absorb large amounts of oil during cooking, which significantly increases their calorific content and reduces the nutritional value.
Fortunately, VA Whitley & Co Ltd, a UK supplier to the Fish and Chip and Fast Food Trade, has perfected a healthier batter which comprises fava bean flour (from the Vicia faba bean) in place of the traditional wheat flour. The fava bean flour has been found to reduce fat/oil uptake during cooking, while importantly maintaining the familiar colour, texture and taste of traditional fish batter.
Not only does this batter reduce the overall calorific content of the resulting food product, but it also meets many of the other consumer-driven demands of today. For example, the use of fava bean flour provides additional health benefits over wheat flour as it is higher in protein, fibre, and trace minerals. Fava bean flour is also gluten-free, and so is suitable for those among us who are either intolerant to or just prefer to opt for gluten-free alternatives.
Low Sodium Salted Treats
It is well documented the consumption of excessive amounts of sodium can produce detrimental effects on the circulatory system, such as high blood pressure, as well as kidney affections, water retention, and stomach ulcers.
While many manufacturers have gone some way to meet the PHE salt reduction targets, this has to be balanced with a strong consumer demand for the flavour and organoleptic qualities of salt, particularly sodium chloride.
In order to reduce the amount of salt required in food products, ConAgra Foods Ltd, a US based food manufacturer, found that a solution to this conundrum is to use salt with a mean particle size of less than 20 microns. This invention is based on the fact that reducing the mean particle size of salt increases the total surface area of salt for a given weight. The larger surface area of salt acting on the tongue, provides the same salt perception to the consumer but with the advantage of lower amounts of salt. ConAgra’s proprietary Micron Salt, can be found in its Orville Redenbacher’s popcorn.
Healthy Chocolate
“Chocolate is a perfect food, as wholesome as it is delicious, a beneficent restorer of exhausted power…it is the best friend of those engaged in literary pursuits.” – Justus von Liebig.
While chocolate is a favourite treat for many of us, an increased consumption of sugar has been linked to the higher levels of obesity
in the UK, with the Health Survey for England 2017 finding that 64% of adults were either obese or overweight. Obesity has in turn been linked to diseases such as type 2 diabetes. One food product often discussed with respect to the increase in obesity is milk chocolate, as over half of the weight of a chocolate bar may be due to sugar alone.
IMMD SP Z OO, a company specialising in the field of biotechnology, has dedicated research into finding ways to make chocolate healthier for consumers. Its patent (WO 2018/087305) describes a chocolate product comprising a polyphenol-rich plant extract, preferably trans-resveratrol (t-RSV).
The incorporation of polyphenols in milk chocolate has great health implications as polyphenols have been shown to interfere with glucose absorption in the intestine. IMMD SP Z OO also states that the chocolate containing polyphenols can provide ‘antioxidant, anti-inflammatory, anti-hypoxic, vascular supporting and/or other health benefits’.
Low-Fat Baked Goods
Over the years, bread and bakery products have become a staple in our diets. However, compared to other food types, baked goods can contain higher calories and higher amounts of carbohydrates, along with providing fewer vitamins and minerals.
One particular type of baked product which is known for its higher calorie content is puff pastry. Puff pastry is formed from a dough laminate containing many alternating layers of the basic dough and a fat. Fat plays an integral role in forming the puffed final product.
During baking, the water contained within the dough is turned into steam, which is trapped by the thin layers of gluten, causing the dough to expand. The fat layers contained in the dough act as a barrier, preventing the gluten layers from joining together. In order to function as an effective barrier layer, the fat used must contain certain levels of plasticity (to enable the dough to be rolled and folded when creating the laminate structure) and firmness (as softer fats can be absorbed by the dough).
While it would be desirable to produce puffed pastry products which do not weigh as heavily on the calorific scales, this problem cannot be solved by simply using less fat or substituting the fat for a softer alternative, containing lower trans fats.
However, AAK AB, a company specialising in vegetable oil and fats, has developed a reduced fat bakery emulsion that enables the preparation of low-fat puff pastry. In order to reduce the fat content of the emulsion, while still maintaining the required plasticity and firmness, the patented bakery emulsion replaces part of the fat traditionally used with natural additives, such as maltodextrin, which mimic the properties of the fat.
Reduced Sugar Soft Drinks
As discussed above, national organisations, such as PHE, have heavily publicised targets for reducing the sugar content of a range of products that contribute most to children’s sugar intake by at least 20% by 2020.
One organisation taking this challenge head-on is Lucozade Ribena Suntory (LRS), a European based company owned by Japanese manufacturing company, Suntory, which has announced that it aims to reduce the sugar content of all of its existing and new beverages to less than 4.5g per 100 ml (compared to previous 10 to 11g per 100 ml). LRS has managed to remove a staggering 25,500 tons of sugar and 98 billion calories from the company’s annual drinks production and states that this dramatic change has not affected the taste of the soft drinks produced.
So, how are manufacturers meeting these new government-led and consumer-driven targets?
One main method of lowering sugars in food is to replace at least some of the sugar present with sweeteners. Unfortunately, the incorporation of higher amounts of sweeteners can lead to the presence of an off/bitter taste. It is known that the presence of artificial sweeteners within food products not only stimulates the human sweet taste receptors, but also activates bitter taste receptors (TAS2Rs), causing this unpleasant ‘off-taste’.
In recent years, LRS has been reformulating its drinks products in order to reduce sugar content while maintaining a sweet taste. One method of achieving this is discussed in the patent of Suntory Holdings Ltd, WO 2018/225817, which discloses a sweetening composition comprising a combination of natural sugars, high-intensity sweeteners and low concentrations of sodium. The invention utilises the fact that the perceived sweetness of a food or drink product is increased by the presence of sodium.
Protecting Market Share
Producing food and drink products which meet all of these requirements can provide companies with a competitive advantage, but the question remains: how can this advantage be maintained and how do companies prevent competitors reaping the rewards from their research and investment?
We see numerous examples of food and drink manufacturers maximising the effectiveness of their IP protection as they develop innovative product ranges and tests to meet fast-moving consumer demand. IP protection can relate to the finished food product, a specific combination of ingredients or the manufacturing process itself, as well as working with design law to protect the aesthetics of products, the food product itself and its packaging.
This article was first published in the May 2019 edition of Intellectual Property Magazine.
Patents provide an alternative and interesting source of information for identifying emerging technology trends. One such recently observed trend supports a growing interest in the use of indirect biomarkers in diagnostic assays. This article describes the key differences between direct and indirect biomarkers, and illustrates two examples to show how indirect biomarkers are providing new clinical diagnostic opportunities, namely to improve initial triaging of patients in accident and emergency scenarios and to allow rapid and accurate screening of patients carrying a latent tuberculosis infection.
Classical diagnosis of disease-causing agents relies on identification (i.e. direct detection) of a key biomarker that is characteristic of said agent. Examples of such biomarkers include: nucleic acids (detection via polymerase chain reaction hybridisation, sequencing, isothermal amplification); antibodies (detection via ELISA, immunofluorescence (IF)); antigens/ proteins (detection via ELISA, IF); and agent isolation (detection via differential cell culture means). This, in turn, requires knowledge of the infectious agent in question (table 1), and goes together with constant refinement pressures to ensure all newly-emerging variants are also captured. The high specificity typically demonstrated by such biomarkers, however, unfortunately presents an Achilles’ heel when a negative diagnostic outcome is reached such that further intelligence (other than subtractive analysis of the targeted agent per se) can be gleaned.
It should also be noted that the different biomarker types demonstrate their own strengths and weaknesses when assessed in terms of, for example, minimum diagnosis time requirements, transportability, relevant detection window timeframe, and sensitivity (table 2).
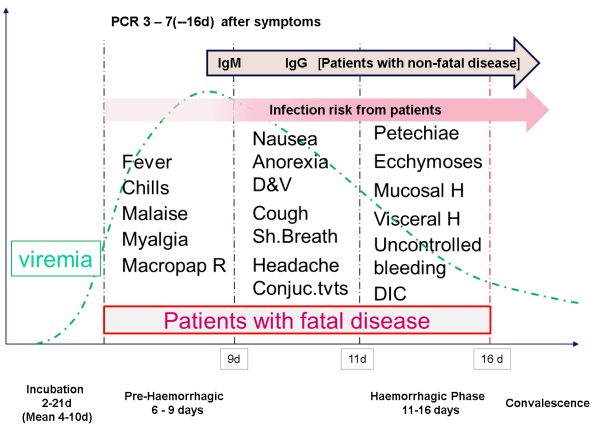
By way of example, the following Ebola case study (figure 1) nicely illustrates the potential criticality of detection window factors that impact both polymerase chain reaction and antibody detection. In more detail, premature use of polymerase chain reaction detection during either the ‘incubation’ or early ‘pre-haemorrhagic’ phases will likely result in a false negative. Similarly, premature use of a diagnostic for IgM up to and including the mid ‘pre-haemorrhagic’ phase would also likely yield a false negative.
Figure 1: Multiple Biomarkers – Ebola case study (source: Prof Nigel Silman, PHE Porton, Salisbury SP4 0JG)
While significant advances have been made with classical biomarker diagnostics, there remains a variety of notable unmet clinical needs:
- rapid, accurate biomarker tests for differential diagnosis;
- biobanks of well characterised clinical
samples;
requisite materials to construct immune-assays and
molecular diagnostics;
true point of care platforms for diagnostic
assays; and
robustness to permit field use.
Is now the time for an alternative (potentially supplementary) approach?
Recent developments with the use of indirect biomarkers (e.g. differential gene expression or metabolism profiles) have attracted significant interest. The use of carefully selected biomarker sets in combination with robust algorithms provide important assay flexibility and allow specificity and/or sensitivity levels to be dialled-up or down as appropriate to achieve an optimal read-out. Moreover, this flexibility provides the means for further interrogation of a clinical scenario where initial diagnosis has failed to identify a positive outcome, and thereby addressing one of the major weaknesses associated with classical biomarkers weaknesses.
By way of example, WO2018/060739 teaches use of a variety of different marker sets can be employed to distinguish between septic inflammatory response syndrome (SIRS) and sepsis, and subsequently to differentiate between important subsets thereof. In more detail, a simple triage diagnostic of this type would find immediate value, for example when assessing a patient who has presented in any emergency scenario. Rapid point of care confirmation that the patient has SIRS would negate the need to administer antibiotics, and potentially represents a huge step forward in improving current antibiotic husbandry.
Similarly, for a patient presenting with sepsis, being able to differentiate between abdominal sepsis and pulmonary sepsis would permit a more informed decision to be made with regard to the type of antibiotic administered (e.g. to target gram positive, or gram negative bacteria). Another interesting biomarker set described allows one to monitor patient recovery and hence to determine more accurately the correct time point for appropriate patient discharge from hospital. This would, of course, help to address current bed capacity problems without compromising patient safety.
By way of further example, WO 2015/170108 teaches use of a variety of biomarker sets for identifying M tuberculosis infection. This infectious cycle of this agent includes an intracellular ‘latency’ phase during which bacilli do not circulate freely within the body, and are therefore particularly difficult to detect. A further setback with the use of classical biomarkers for detecting M tuberculosis is skin testing may be compromised by BCG vaccination and by exposure to environmental mycobacteria. The presence of a large reservoir of asymptomatic individuals latently-infected with mycobacteria presents a major problem for global control of M tuberculosis infections. This is indeed a key priority for many health and immigration authorities, particularly at the ‘point of entry’ for developed countries where the majority of TB cases are imported. The indirect biomarker sets described achieve both accurate and timely diagnosis of early-stage infection where conventional biomarker detection (via cell culture or PCR) is not possible. Possibly of most significant interest, the authors describe use of unique marker sets that provide robust diagnosis of latent M tuberculosis at sensitivity and specificity levels far superior than have been hitherto reported.
These two brief case studies illustrate the huge opportunities indirect biomarkers present for rapid bio-sensing technologies for both the control and prevention of infectious diseases, and related heath- or life-threatening management scenarios.
We urgently need to start moving away from sole reliance on classical biomarkers (noting in particular the impotence of these markers in negative diagnostic output scenarios) and start embracing the new world of indirect biomarkers to detect and characterise important host signature profiles.
This article was first published in the April 2019 edition of Intellectual Property Magazine.
Nobody can fail to notice the ever-increasing presence of the internet in almost all aspects of daily life, with seemingly everything now having some level of connectivity. Medtech is clearly no exception and in fact, is one of the technology sectors to most embrace the opportunities that Internet of Things (IoT) brings – with the interconnection of devices and systems via the internet (IoMT). However, innovating in the IoMT world may involve stepping into other areas of technology that are different to those with which the medtech community are familiar, bringing new challenges, new competitors, and the need for a new way of thinking, especially with regards to your intellectual property (IP) strategy.
In recent years, medtech companies have been developing ways to enhance products using the IoT. Just a few examples are wearable monitors that send data to a connected device or to a healthcare provider; smart medications that record how and when they are used and provide feedback for improving efficacy; data tags in manufacturing and packaging to monitor batch quality, shelflife, shipping and returns, etc. Each of these, and the many other applications being developed, requires transfer of data between devices.
A mistake I have often seen made in the medtech sector, even by otherwise IP savvy companies, is incorporating known communications technologies into devices without checking whether this is covered by existing IP. Whilst it may seem surprising, there is often problematic IP held not only by known competitors (whose own IP strategy you may be comfortable with), but more frequently by companies with whom you previously did not compete, since many big-name IT companies are now moving into the medtech space, and they may have a very different approach to IP. Should they enforce their IP rights, it can be very costly and damaging to a business, to the point you may have to remove your product from the market, and the accompanying compensation payable to them may be significant.
Therefore, it is important to rethink and modify your IP strategy if you want to take advantage of the IoT. One of the biggest changes I recommend is to consider the IP landscape at the earliest stage in development and as frequently as possible, since the technology sector is very fastpaced (compared with MedTech) and new IP can arise even when you think you have cleared a product. Tech companies are extremely prolific when filing IP, so it can often seem like there is a huge amount of IP covering what you want to do and no way through it. However, much of this IP is speculative and unlikely to be valid in broad terms, so its existence does not necessarily prevent you from doing what you want, but you will need clear, practical advice to help you make strategic decisions.
Another aspect that is quite often neglected by medtech companies is filing your own IP. Medtech is generally a very innovative sector, with developments that stand out as being inventions. Therefore, it’s common for medtech companies to see communications technologies as not adding anything particularly innovative to a product and to not seek protection for the new IoMT device. You are missing out on valuable protection that not only can be used to stop competing products, but can also be a bargaining and commercial tool should you find yourself within the IP protection of another company and in need of a licence for example.
Another area that must be considered is data protection, especially with the recent legislation changes such as the EU General Data Protection Regulation (GDPR) which came into force in 2018. How you implement GDPR may lead to IP opportunities and again, commercial opportunities with regards to licensing.
Timely and appropriate IP advice throughout your development cycle is essential. You can spend large amounts and still not avoid the pitfalls, whereas getting it right from the very start can save costs over time. As you are moving into another sector, with big name players that are typically more aggressive with their IP, and whilst it can be daunting, the rewards can be significant. As Facebook’s Mark Zuckerberg said: “In a world that’s changing really quickly, the only strategy that is guaranteed to fail is not taking risks”.
This article was first published in the March/April 2019 edition of Med-Tech Innovation Magazine.
Mathys & Squire will be exhibiting on stand F58 at the Med-Tech Innovation Expo on 15-16th May.
Why is Facebook Suing Over Trade Mark Infringement and Cybersquatting?
Legal action and Facebook is nothing new, but why – and who – is the social media giant suing over trademark infringement and cybersquatting? At the start of this month, Facebook released a short blog post confirming it was taking legal action against a small cluster of Chinese websites. This legal move was prompted by the fact that these websites were selling fake accounts, followers and likes on Facebook and its sister company Instagram.
In the blog post entitled Cracking Down on the Sale of Fake Accounts, Likes and Followers, the company said it was enforcing its “rights under US intellectual property law” based on the websites “illegal use” of its trade marks and brand. It concluded by saying: “Inauthentic activity has no place on our platform. That’s why we devote significant resources to detecting and stopping this behaviour, including disabling millions of fake accounts every day. Today’s lawsuit is one more step in our ongoing efforts to protect people on Facebook and Instagram.”
What is Trade Mark Infringement?
Trade Mark infringement is the unauthorised use of a trade mark by a company offering a related or competing service or product. That being said, it’s important to know that a trade mark need not be identical to constitute an infringement. It comes down to whether the use of the trade mark is likely to confuse consumers. In this case, the explicit use of the word ‘Facebook’ in the domain names, along with the fact that the sites were offering fake accounts, likes and followers (a breach of Facebook’s terms of service) was deemed sufficient for the company to decide to take action.
What is Cybersquatting?
Cybersquatting is defined under US federal law as the practice of registering, selling or using a domain name with the intention of profiting from a trade mark belonging to someone else. In the case of this particular lawsuit, Facebook is alleging that the companies in question are using its trade marks by using web addresses such as ‘facebook88.net’, ‘myfacebook.cc’ and ‘infacebook.cc’. By using the ‘Facebook’ name, these sites are attempting to profit from the goodwill and reputation that has been built up in the brand.
The Facebook Lawsuit
The lawsuit that Facebook has filed alleges that four Chinese companies and three individuals based in China have been operating a number of websites since 2017, and that by promoting the sale of bogus accounts it is infringing Facebook’s trade marks and terms of service. The companies implicated are Xiu Network (Shenzhen) Science and Technology Company, Xiu Feishu Science and Technology Company, Xiufei Book Technology Co. and Home Network (Fujian) Technology Co. Ltd.
Facebook is asking for the companies to be banned from creating misleading sites and fake accounts, and for the profits derived from the activity to be awarded to Facebook along with $100,000 for every infringing domain name.
The lawsuit is an unusual move for the company, which already purges millions of fake accounts every day; 2.1billion were disabled between January and September 2018 by its own account. So, the move to file a lawsuit is likely due to the extent of infringement in this particular instance. Regardless of the outcome, other companies operating in the same manner would be wise to keep a close eye on the verdict. In issuing of the lawsuit, Facebook is sending a clear message that illegal activity will not be tolerated.
Mathys & Squire is one of Europe’s most highly regarded intellectual property firms. Contact us to find out how we can help ensure your business grows legally, and how we can help enforce your intellectual property rights.
Chimeric antigen receptors (CARs) are artificial proteins that are used to redirect immune system cells to attack targets that are normally invisible to them, such as cancer cells. CARs are typically made by fusing the antigen binding domain of an antibody specific to a target of interest to the transmembrane and intracellular signalling domains of receptors normally found on the surface of immune cells. When a T-cell or other immune cell expressing the CAR encounters the target, the CAR binds it and stimulates the cell to attack.
Interest in CARs has rocketed following reports a few years ago that patients with refractory blood cancers were achieving complete remission after CAR cell therapy. Even a single dose of CAR cells can be highly effective, and for a very long period of time, because unlike conventional drugs, CAR cells are able to live on and patrol inside the body, much like normal immune cells. Due to its impressive effects in treating blood cancers, an anti-CD19 CAR-T therapy called Kymriah (tisagenlecleucel) became, in 2017, the first genetically modified cell therapy approved by the FDA. The Kymriah approval was shortly followed by FDA approval of a second anti-CD19 CAR-T therapy called Yescarta (axicabtagene ciloleucel). Both drugs gained approval in Europe in 2018, and it is likely that more CAR cell therapies will be approved, particularly in relation to blood cancers.
Current Challenges
One of the main challenges of CAR cell therapy is manufacture. ‘Foreign’ cells are normally rejected by the host immune system, so it is important to manufacture CAR cells that are a suitable match to the recipient. In the case of Kymriah and Yescarta, this is achieved by using the patient’s own T-cells to engineer the CAR cell therapy they are going to receive. Both drugs are thus a form of ‘personalised medicine’, the manufacture of which for patients in different countries presents a logistical challenge that increases the cost of therapy. In the US, for example, the list price of Yescarta is reported to be $373,000.
Patent Landscape
The growing interest in CAR-T therapy has been accompanied by massive growth in the number of CAR-related patent filings, see figure 1.
The biggest PCT filers by applicant name include Cellectis, Juno Therapeutics, Novartis, the University of Pennsylvania, and the US Department of Health and Human Services. Juno, which has been acquired by Celgene, has a candidate anti-CD19 CAR-T therapy called JCAR017, and Celgene has a candidate anti-BCMA CAR-T therapy called bb2121 (developed in collaboration with Bluebird Bio), both of which are in clinical testing against blood cancers. Novartis owns the Kymriah product, which it developed in collaboration with The University of Pennsylvania. Other significant players include Kite Pharma, now part of Gilead, which owns the Yescarta product. Both Kite and Novartis also have candidate anti-BCMA CAR-T therapies that are undergoing clinical evaluation.
The patentability of CAR cases is often based on aspects of CAR design. The choice of target antigen or binding domain may give rise to patentability, much like in conventional antibody cases. Other design aspects include the choice or combination of transmembrane and intracellular signalling domains, and the format of the CAR, which may be presented e.g, as a single molecule or as a split structure in which the target binding and intracellular signalling functions are partitioned into separate molecules that can associate.
Patent protection can also be sought for aspects of CAR cell manufacture. For example, Gilead highlights Kite’s European and US manufacturing patent applications as relevant to its Yescarta product. Particular medical applications of CAR cells may also form the basis of patentability.
With an increasingly crowded patent landscape comes litigation. Kite has already tried – unsuccessfully – to invalidate a US patent relating to Sloan-Kettering’s CAR technology, and further disputes seem inevitable.
The Future
It is likely that approval will soon be given for the treatment of further forms of blood cancer using CAR cell therapy, and it is hoped that this new class of drugs will also prove effective at treating solid tumours. Much research effort is also going into the development of ‘universal’ CAR cells – an off-the-shelf product that does not require personalised manufacturing. Cellectis and Celyad both have universal CAR-T therapy candidates in clinical testing.
This article was first published in the March 2019 edition of Intellectual Property Magazine.
In 2016, a group from academia, industry, funding agencies, and scholarly publishers produced a paper on how the infrastructure supporting the reuse of scholarly data could be improved, with the underlying objective of extracting the maximum benefit from research investment.
The paper outlined the FAIR guiding principles for scientific data management and stewardship: findability, accessibility, interoperability and reusability. The principles aim to bring clarity to the goals of good data management and stewardship, and to define simple guideposts to inform those who publish or preserve scholarly data.
One of the key benefits in increasing the availability of data is that it helps improve its accuracy. For instance, data from different studies may be combined to create large data sets for analysis by the scientific community. It will also be subject to greater levels of peer review thereby allowing any assumptions used in creating the data, or conclusions that are drawn from it, to be informedly challenged.
While an increase in the accuracy of data is undoubtedly a good thing for the scientific community, there is a question of whether improvements in the availability of data will inevitably lead to innovators losing control of their intellectual property.
What Are the FAIR Principles?
The original 2016 paper elaborates on what is meant by findability, accessibility, interoperability and reusability. However, one of the clearest interpretations has been set out by the Association of European Research Libraries (LIBER), an early endorser of the principles. According to LIBER:
- findability requires that data and supplementary materials have sufficiently rich metadata and a unique and persistent identifier;
- accessibility requires that data and metadata are understandable to humans and machines. Data is deposited in a trusted repository;
- interoperability requires that metadata use a formal, accessible, shared, and broadly applicable language for knowledge representation; and
- reusability requires that data and collections have clear usage licences and provide accurate information on provenance.
What is absolutely clear from the original 2016 paper is that the FAIR principles are intended to apply not only to ‘data’ in the conventional sense, but also to the algorithms, tools, and workflows that led to that data.
And What Is Their Impact?
The FAIR principles reflect the movement in recent times towards open science values. Many of the FAIR principles had already been adopted in the scientific community.
In the original 2016 paper, the following initiatives were highlighted as examples of systems in which at least some of the FAIR principles were already being implemented: Dataverse (an open-source data repository software), FAIRDOM (integrating the SEEK14 and openBIS15 platforms in a FAIR data and model management facility for systems biology), ISA16 (a metadata tracking framework to facilitate collection, curation, management and reuse of life science datasets), Open PHACTS (a data integration platform for information pertaining to drug discovery), wwPDB (an intensively-curated data archive about experimentally-determined 3D structures of proteins and nucleic acids) and UniProt (a comprehensive resource for protein sequence and annotation data).
Since the publication of the original 2016 paper, the term ‘FAIR’ has been gaining traction.
The European Commission is establishing the European Open Science Cloud (EOSC), an initiative to provide a digital infrastructure that brings computing and data storage capacity to scientists across the European Union. As part of this initiative, the Commission in 2018 published a report entitled Turning FAIR into reality. One of the suggestions for funding a FAIR data system is the introduction of a requirement that a certain percentage e.g. 5%, of funding be allocated towards managing and stewarding data.
If initiatives such as the EOSC are implemented, then it seems likely that the FAIR data principles will be adopted in the scientific community on a large scale. This will enable ‘old’ data to be innovatively reused, thereby maximising the value of the original research investment and paving the way for developments in fields in which large, accurate data sets are of paramount importance such as personalised medicine and diagnostics.
Compatibility
Patenting is compatible with FAIR principles. Many open science resources are open in the sense that they are non-discriminatory, i.e. anyone can access and use the information that they contain, but nonetheless require the user to take a licence. Patents and other intellectual property rights can be hugely useful in defining the framework of the licence.
For instance, the Biological Innovation for Open Society (BiOS) initiative makes technology covered by certain patents – e.g. the TransBacter™ biological gene transfer system for eukaryotic cells – available for non-exclusive use by any entity that agrees and conforms to the terms of a BiOS licence. The licence allows both research and commercial use but encourages sharing of improvements among all licensees.
Rather than frustrating the open science movement, intellectual property rights may, therefore, be seen as a key instrument for promoting and propagating the FAIR principles of data management and stewardship.
This article was first published in the March 2019 edition of Intellectual Property Magazine.
In Actavis Group PTC EHF and others v ICOS Corporation and another [2019] UKSC 15, the Supreme Court had to consider whether claims directed to a new dosage regime of a drug were obvious. The patent (EP 1173181) was owned by ICOS Corporation and was the subject of an exclusive licence to Eli Lilly & Co. Its claims (including second medical indication claims in both the ‘Swiss-style’ and the newer ‘EPC 2000’ format) covered a dosage regimen of up to 5 mg per day for the compound tadalafil, sold under the brand name Cialis for the treatment of erectile dysfunction. Actavis sought to invalidate the patent on the basis of ‘Daugan’, which was the first medical use patent for tadalafil.
At first instance the judge had held that the dosage regime was non-obvious, but this was overturned on appeal. ICOS now sought to reverse the decision of the Court of Appeal.
The Supreme Court upheld the Court of Appeal’s finding of obviousness. In reaching this conclusion, the court listed 10 factors which were ‘relevant considerations’, including whether at the priority date something was ‘obvious to try’ and whether the research leading to the invention was of a ‘routine nature’, as well as whether the results of the research were surprising.
Although ICOS had argued that it was surprising to find that a dosage of 5 mg was effective and associated with reduced side-effects, the court nevertheless agreed with the Court of Appeal that this merely lay “at the end of the familiar path through the routine pre-clinical and clinical trials’ process” given the context of the routine Phase IIb dose-ranging studies in which the dosage had first been tested. Once the dosage had been identified via routine studies, any ‘surprising’ effects associated with it were merely an ‘added benefit’ and not sufficient to confer an inventive step. The court did not provide any guidance as to how the various ‘relevant considerations’ should be weighted, stressing that this would depend on the facts of any particular case. It also explicitly recognised that, in principle, valid dosage regime patents may exist. However, it seems that in many cases dosage regime patents may now be found invalid in the UK unless (for example) there is something unusual or non-routine about the circumstances surrounding identification of the dosage.
Food and drink companies face a multitude of challenges in their quest to attract and retain customers. In the search for a competitive edge, companies invest heavily in research and innovation in order to produce items with the requisite taste, mouthfeel, appearance and nutritional value whilst simultaneously controlling production costs. In addition, there is the added pressure of changing consumer trends, such as the rise in vegetarianism and veganism, alcohol-free drinks, and healthier versions of comfort foods, whilst meeting new governmental policies and requirements: quite the raw deal.
The ability to meet such requirements can provide companies with a competitive advantage. The question then is how can they maintain this advantage, and how can they prevent competitors reaping the rewards of their research and investment?
There are typically two methods used within the food and drink industry to protect intellectual property: trade secrets and patents. Trade secrets can be useful where it is difficult (if not impossible) to derive the ingredients or process used to produce the food or drink product.
However, trade secrets provide no protection if another company legitimately produces the same product, manufacturing process or simply reverse engineers the product produced.
Patents may therefore provide a better form of protection where it is possible to derive the recipe (or method of manufacture) from the food or drink product itself e.g. where a recipe or composition could be determined by simply analysing the end product.
So, what can be protected within the food and drink industry; and what steps can you take to protect the IP on your plate?
What Can Be Protected Within the Food and Drink Industry?
Suitable food and drink products may include those with an improved taste, texture or appearance whilst reducing fat or sugar content; combinations of ingredients producing a synergistic effect; a non-obvious substitution for a commonly used ingredient (e.g. a reduction in E-numbers); and methods of altering the flavour profile of food and drink products, to name just a few examples of eligible contenders.
Processing methods within the food and drink industry can also be protected, whether these relate to more cost-effective manufacturing methods; methods of providing improved mixing of ingredients; or new process steps which provide an unexpected result in the product. Given the increased desire to produce environmentally friendly products, new environmentally friendly or biodegradable packaging may also be patentable.
How Can Patents Help?
A patent is an intellectual property right granted by a specific country’s government for a limited time period, typically 20 years from the filing date. A patent enables the owner to prevent third parties from making; using; offering for sale; selling; keeping; or importing the patented invention within the territory for which the patent has been granted. For a specific process, the owner has the right to prevent third parties from using or offering for use that process within the relevant territory without the patent owner’s consent.
What’s the Best Protection For Your Invention?
Before filing a patent application it is advisable to review your business strategy and consider how your IP ties into your commercial aims.
Patenting inventions can be a costly process, especially as the number of territories in which you require protection increases, and so it is worth considering which territories can most effectively support your business. For example, where are you planning to sell your product or use the process?
If you do not wish to exploit the IP yourself, where might you wish to licence the product/process? In considering the actions of third parties, are there particular counties in which you wish to prevent a competitor from exploiting your invention?
Applications in Europe can be filed via a European patent application at the European Patent Office (EPO) enabling patent protection in up to around 40 countries on the basis of a single application. An alternative route is the Patent Cooperation Treaty (PCT) which covers over 100 countries in a single initial patent application, and allows delayed selection of countries such as US, India, China and Japan as well as the EU.
What Criteria Do I Need To Meet In Order To Get a Patent Granted?
In general, to obtain a patent, the IP must be novel, inventive and capable of industrial application.
Novelty
A claimed invention cannot have been publically disclosed before the date on which the application was filed. The assessment of novelty can be based on any public disclosure including scientific journals, published articles, presentations, sale of the product itself or displaying the product at a trade show (where it would be possible for someone to determine the novel features from the product itself). For this reason, it is essential that no details of the invention are publically disclosed before the date on which the patent application is filed. However, if disclosure is unavoidable, for example in investment meetings, it is advisable that non-disclosure agreements (NDA) are used.
Inventive Step
The claimed invention must also be inventive, i.e. not obvious in view of what was known at the filing date of the patent application. The assessment of whether something is inventive is based on the knowledge of a skilled person within that field.
Industrial Application
The claimed invention must be capable of exploitation within an industry. Most products and processes within the food and drink industry will meet this criterion.
After Having Met These Three Requirements, What Are Your Next Steps?
Filing a Patent Application
A patent application contains a description of the invention for which patent protection is sought and typically includes:
• a discussion of the background art;
• a statement of invention;
• examples;
• claims; and
• any relevant figures.
The background discussion outlines what was known in the field at the filing date of the application and any issues associated with the known products, processes and uses. If the claimed invention suggests its use will overcome or at least provide an improved effect on existing products, it is useful to know what existing issues there are.
The statement of invention defines the claimed invention. This section defines each feature of the claim and may also discuss possible alternatives to these features.
Examples are often provided to illustrate that the claimed invention can be put into practice. Where a particular advantage associated with the invention has been discussed, for example an improved taste with reduced sugar content, the examples can illustrate the improved effect compared to previous products.
The claims define the boundaries of the invention for which protection is sought. Typically, the first claim broadly defines the invention in order to obtain the broadest scope of protection possible.
Subsequent claims further define the features of the invention or include additional features, thereby narrowing the scope of protection.
Figures can also be included. These can include graphic illustrations of the claimed invention, flow diagrams illustrating a particular process or graphical representations of data produced through the analysis of the claimed product (or a product produced by a claimed process) compared to products and processes already known in that field.
Once the application has been filed, it is generally not possible to amend the application to redefine the invention, include addition information or correct errors. As such, if the application is poorly drafted, it can leave the applicant with little or no protection for their invention. The drafting of the patent application can therefore be almost as important as the invention itself and so it is always recommended to hire a patent attorney with the requisite qualifications and experience to ensure that the application is of value to your business.
Examination Of the Patent Application
After filing, the application is searched and examined by a Patent Office Examiner. If the Examiner does not consider the patent application to meet one or more of the criteria required to grant a patent, a report detailing the objections raised is issued. One of the main roles of a patent attorney is to assess any objections raised by the Examiner and advise how best to address the objections raised, particularly in light of your business strategy.
Once a response has been filed, the Examiner reassesses the application. If the Examiner considers the application to now meet the necessary requirements, a patent will be granted, if not the Examiner will issue a further report.
So Your Patent Is Granted: Then What?
It is a common belief that once a patent has been granted, there is no bar from manufacturing and selling the claimed product or using the claimed process – this is not always the case! A patent is a negative right, meaning that only allows you to prevent third parties from doing these acts within the territory for which the patent has been granted. It is possible that there may be other third-party patents which could prevent you using your product or cover upstream or downstream processes which you are planning to use.
Accordingly, before commercialising your product or process it is advisable to consider such third-party rights. This can be done by requesting a freedom to operate search (FTO). If any potentially relevant patents or applications are found, a patent attorney can analyse these documents in view of your commercial products and/or processes. If these documents are considered relevant it may be possible to design around them; seek a licence from the rights holder; seek to invalidate the third-party rights or even, depending on the age of those rights, simply wait for them to expire.
If you believe you have developed valuable commercial IP, it is highly advisable to consult a patent attorney for more detailed information on identifying and protecting the intellectual property in your innovation.
This article was first published on New Food Magazine in March 2019.
In a recent High Court decision (Eli Lilly & Company vs Genentech, Inc), Mr Justice Arnold referred the following question to the CJEU: “Does the SPC Regulation preclude the grant of an SPC to the proprietor of a basic patent in respect of a product which is the subject of a marketing authorisation held by a third party without that party’s consent?”
The referral arises in the context of Genentech’s European Patent EP1641822 B1 and Lilly’s marketing authorisation (MA) EU1/15/1085 for Taltz® (ixekizumab) – Genentech pursued a supplementary protection certificate (SPC) based on its EP patent and with reference to Lilly’s MA.
The referral in this case may come as a surprise to many, since the practice of applying for ‘third party SPCs’ is now relatively common, and is usually accepted by national patent offices. This practice follows an earlier CJEU decision (C-181/95 – Biogen) which indicated that the SPC Regulation did not require there to be a relationship between the holder of the basic patent and the holder of a related MA.
However, the practice of applying for ‘third party SPCs’ without the consent of the MA holder can be problematic – the MA holder must then either take a licence under the SPC, or seek to invalidate the SPC, in order to commercialise the product for which they have obtained the MA. In view of this, the practice is not without criticism and, moreover, comments from both the CJEU and the Advocate General (in C-493/12 – Lilly and Teva v Gilead, respectively) have implied that an SPC should only be granted to a party investing in research relating to the authorised product.
Parties will now discuss the wording of the question before a final referral is made to the CJEU.
The outcome of this referral will be of significant interest to the biotech community and may have an impact on patent proprietors seeking ‘third party SPCs’, particularly if no legal relationship between the parties exists and/or if consent from the MA holder has not been obtained. We also note that the referral question has interesting parallels to the paediatric extension provisions recently enacted in Switzerland, which appear to suggest that the documentation required in order to apply for a paediatric extension of a Swiss SPC may only be requested from Swissmedic by the MA holder.
If you have any questions about this article, please contact Jonathan Israel for more information.