“Protecting the expectations of third parties rather than sanctioning the applicant for its negligence is the primary consideration” – re-dating the withdrawal of an application in order to deem a divisional application validly filed.
In J 5/19, the EPO’s Legal Board of Appeal took a relatively lenient decision on 21 January 2021 in favour of an applicant regarding an erroneous act by their representative. The European representative had received two emails from a US attorney representing the applicant, the first providing instructions to file a divisional application prior to the deadline for filing a response to a Communication pursuant to Article 94(3) EPC issued with respect to the parent application. The second email requested the parent application be withdrawn or abandoned. However, the representative performed these acts in the wrong order – the letter withdrawing the parent application was dated 3 May 2018 and the divisional application was filed on 8 May 2018. Both of these acts appeared on the register on the same date, 11 May 2018.
A request to ‘retract’ the withdrawal of the application, citing Rule 139 EPC, was filed on 29 May 2018. The representative argued that the filing of the withdrawal was a mistake and the true intention had been to file a divisional application and subsequently withdraw the parent. The Examining Division rejected this request on the basis that “according to the case law of the boards of appeal, a withdrawal could not be retracted once it had been entered into the register”.
However, the Legal Board of Appeal ultimately overturned this decision, stating that the withdrawal of the application would be corrected so as to be dated between 9 May and 20 May 2018 – i.e. after the filing of the divisional application but prior to the deadline to file a response to the Communication under Article 94(3) EPC issued with respect to the parent.
In forming this decision, the Board held that three cumulative conditions had to be met for the request to be allowed (referencing G 1/12):
- The withdrawal did not reflect the intention of the applicant – the Board makes an important distinction between the assessment of the intentions of the applicant and those of the representative, with the former being the relevant factor in the situation at hand.
- There was no undue delay in seeking the correction – the request to retract was filed only one week after a communication indicating that the second application could not be treated as a divisional application.
- Third parties viewing the file would have had reason to suspect that the withdrawal was erroneous – in other words, the legal certainty of third parties was not impacted significantly, given that third parties could practice an invention after publication of a (non-erroneous) withdrawal.
With respect to point iii., the Board reasoned that as the register indicated that a divisional application had been filed, third parties would understand that the subject matter of the original application could still be pursued through the divisional in one of two scenarios; either the withdrawal was an error (correctable under Rule 139 EPC) or the divisional application was validly filed (under Article 76 EPC). It was considered that a third party could not reasonably rely on either scenario to indicate that the invention was no longer the subject of a pending application, and so the Board held that, in the present case, each of the above conditions had been met.
It is worth noting that, an earlier request of the applicant to reinstate the parent application, was not considered allowable, as this request did not represent the intention of the applicant.
The European Commission has opened a public consultation on legislation relating to plants produced by certain genomic techniques. The consultation is open for submissions until midnight (CET) on the 22 July 2022.
The public consultation is aimed at gathering views and evidence from public authorities, citizens, and stakeholders (both inside and outside of the EU) relating to:
- the functioning of current genetically modified organisms (GMO) legislation; and
- how best to regulate plants obtained by targeted mutagenesis and cisgenesis.
The consultation is particularly aimed at those in the fields of agriculture, food and feed, plants, the environment, sustainability, biotechnology in general and the application of targeted mutagenesis and cisgenesis in plants, including their food and feed products.
In 2018 the European Court of Justice (CJEU) ruled that the legal regulations for GMOs applied to all organisms which have been altered using new genome editing methods. At the time of the ruling, many innovators in this space questioned the scientific basis for CJEU’s ruling and warned that this would make the research and development of improved crops to address urgent concerns such as climate change and sustainable agriculture more difficult.
Pleasingly, the European Commission now appears willing to reopen consideration on the important issue of new genomic techniques and adapt the current GMO legislation to the scientific and technological progress of GMO. The public consultation is part of a European Commission initiative to propose a legal framework for plants obtained by targeted mutagenesis and cisgenesis and for food and feed products produced from said plants. The initiative aims to provide an appropriate regulatory oversight for said plants and products which ensures a high level of protection of human and animal health and the environment, while also enabling innovation, particularly in the field of agri-food. An important consideration of the initiative is to enable these genomic techniques to contribute to the objectives of the Farm to Fork Strategy and the European Green Deal.
Following completion of the public consultation, the Commission will conduct an impact assessment. Publication of the impact assessment and of a potential legal proposal is expected in the second quarter of 2023.
The European Patent Office (EPO) has a growing body of case law relating to the significance of clinical trial protocols when assessing novelty and inventive step. The latest in this line of decisions is T 2963/19. Here, an EPO Technical Board of Appeal considered whether a prior art document disclosing a clinical trial protocol without results provided a reasonable expectation that the treatment under investigation would be safe and effective.
The patent in question claimed a combination therapy for treating pancreatic cancer. The claimed dosage regimen involves administering defined amounts of liposomal irinotecan, 5-fluorouracil and leucovorin.
The patent was opposed on the ground of lack of inventive step (among others). The Opposition Division held that the claimed subject matter was obvious in light of a published clinical trial protocol describing a randomised, open label Phase III study of liposomal irinotecan in combination with 5-fluoruracil and leucovorin for treating patients with metastatic pancreatic cancer. The combined dosage regimen disclosed in the published clinical trial protocol differed from the claimed dosage regimen insofar as the order of administration was not specified and the dose of leucovorin was different. In addition, the published clinical trial did not disclose any results. During appeal proceedings, the question of obviousness essentially turned on whether the skilled person would have had a reasonable expectation that the published clinical trial protocol would have been safe and efficacious given the lack of results.
According to the established case law of the Boards of Appeal, a course of action (for example, conducting a clinical trial according to a given protocol) can be considered obvious if the results are clearly predictable or when there is a reasonable expectation of success (T 149/93).
The idea of what constitutes a ‘reasonable expectation of success’ takes on a specific meaning when talking about clinical trial disclosures, such as published protocols. This is because a published clinical trial protocol will generally lead to an ‘expectation of success’. Clinical studies are, of course, built on extensive in vitro and in vivo pre-clinical studies. Additionally, clinical trials must go through an approval process which typically involves peer review and ethical approval. During the approval process, consideration would be given to the likely benefits of the treatment and any potential risks. Therefore, a published clinical trial protocol would generally lead to an ‘expectation of success’ (see, e.g., T 239/16). Indeed, as reported recently in T 96/20, the Board considered that the disclosure of a Phase II clinical trial protocol provides, in and of itself, the skilled person with a reasonable expectation that the treatment would be a success, unless there was evidence to the contrary in the state of the art. These decisions appear to set a high bar for establishing inventive step over the prior disclosure of a clinical trial protocol. When a claim is alleged to lack inventive step based on the prior disclosure of a clinical trial protocol, inventiveness can be established by demonstrating that a known prejudice, e.g., a widely held but incorrect opinion of a technical fact, needs to be overcome (see, for example, T1212/01).
Perhaps unsurprisingly, the stage of the clinical trial is of significance. For example, in T 715/03 the disclosure that a compound is undergoing a Phase II clinical trial was only considered to be indicative of a therapeutic effect if results were also provided. Here, the Board held that a Phase II trial requires the demonstration of an acceptable safety profile in a Phase I trial, but not therapeutic efficacy. In contrast, since Phase II trials are intended to investigate efficacy as well as safety, a document disclosing a Phase III clinical trial may well be considered an implicit disclosure of therapeutic efficacy.
In the present case, the Board of Appeal held that the mere fact that a prior art document had reported the testing of the dosage regimen in a Phase III clinical trial does not automatically provide the skilled person with a reasonable expectation that the treatment under investigation would be safe and efficacious. Instead, with reference to T239/16 and T2506/12, the Board held that an expectation of success was highly dependent on the facts of the case in question. In this particular case, the Board concluded that there would be no automatic expectation of success based on the known challenges associated with developing a therapy for pancreatic cancer.
Instead, the disclosure of the clinical trial protocol had to be assessed in the context of any other disclosure relating to the safety and efficacy of similar dosage regimens. In the case in question, the clinical trial protocol had been preceded by positive reports of similar treatment regimens comprising the administration of liposomal irinotecan (MM-398), 5-fluorouracil and leucovorin in a Phase I setting. In light of these disclosures, the Board held that a positive outcome of the disclosed clinical trial could reasonably be expected.
Furthermore, the Board noted that the patentees themselves had relied upon prior disclosure of successful treatment using the triple combination of non-liposomal irinotecan, 5-fluorouracil and leucovorin in their arguments for sufficiency and plausibility. Given that the patent had proposed the claimed dosage regimen to be safe and efficacious on the basis of these prior disclosure, the Board stated that the same considerations had to apply when determining whether a positive outcome could reasonably be expected from the published clinical trial document. As such, the subject matter of the patent was considered to be obvious.
Outlook
In summary, T 2963/19 highlights the potential risk posed by clinical trial protocols to the obviousness of a medical use claim. It is clear from the growing body of case law surrounding this topic that the potential risk of a clinical trial protocol will be highly fact specific. In particular, whether a clinical trial protocol provides an expectation of success will likely depend on whether there are significant safety concerns or other prejudices relating to the protocol, or for a claim to a combination therapy, whether are there known, effective treatments involving one or more of the recited drugs.
Data provided by Mathys & Squire has featured in an article by The Times, Tech Register, World Intellectual Property Review, Institute of Export and International Trade and City A.M. highlighting a surge in trade mark oppositions following Brexit.
An extended version of the release is available below.
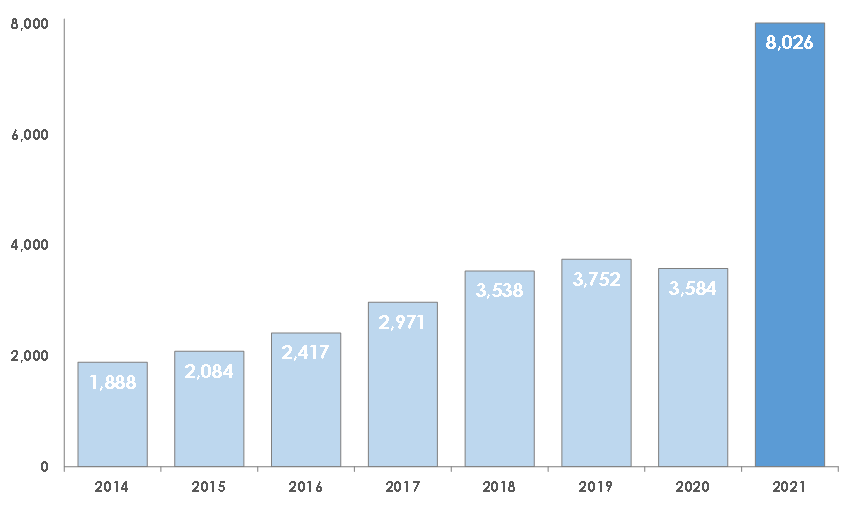
The number of oppositions to UK trade mark applications has more than doubled to a record high of 8,026 in 2021, up from 3,584 in 2020* says leading intellectual property law firm Mathys & Squire.
Mathys & Squire explains that the rise in disputes over UK trade marks has been driven by Brexit. The UK left the EU trade mark system in January 2021, meaning that any business wishing to protect a trade mark in the UK now needs to make a separate application in the UK. This has caused a major rush to file trade mark applications, resulting in a surge in the number of oppositions being filed.
Earlier this year research from Mathys & Squire found there had been a record number of applications for trade marks, with 195,000 applied for in 2020/21, up 54% from 127,000 in 2019/20**.
The Intellectual Property Office (IPO), the government agency that handles trade mark, patent and design registrations, was recently forced to recruit more than 100 new staff to clear a backlog of trade mark applications. This elevated level of trade mark applications – and disputes related to them – is likely to be a long term trend as the UK’s departure from the European Union trade mark system is a permanent one.
Disputes over trade marks, known as trade mark oppositions, occur when a business files a trade mark application with the IPO and another person or business attempts to ‘block’ it. The IPO will determine in opposition proceedings whether a trade mark application should be refused on the basis of an earlier right or other grounds such as bad faith.
Recent disputes over well-known trade marks in the UK have included:
- Amazon opposed an application by a Dubai-based coffee producer covering a range of food and drinks products a similar mark.
- McDonald’s opposed an application to register the trade mark ‘McVegan’ in the UK – it won and was awarded costs.
- Shine TV, the producer of the BBC’s MasterChef TV series, successfully opposed three applications for marks incorporating the words “MASTER CHEF ACADEMY” for education and training services.
Harry Rowe, Managing Associate at Mathys & Squire, comments: “The Brexit-fuelled dash to file trade marks in the UK has inevitably led to more disputes. Businesses need to ensure that they police the register to maintain the distinctiveness and value of their brands.”
“It is likely that this is no short-term spike in disputes – this is what trade mark protection in the UK is now going to look like.”
“Brexit has opened up a whole new battlefield for businesses with valuable brands to protect. Prior to Brexit, trade mark owners could protect their trade mark across all the EU member states in one application. Now that the UK is no longer covered in an EU trade mark, trade mark owners must file two separate applications in order to achieve the same protection.”
“There is now twice as much ground to cover for businesses seeking to protect their investment in their brands.”
Number of trade mark oppositions in the UK surges post-Brexit
* Year end December 31 2021. Source: Intellectual Property Office
** Year end October 31 2021. Source: Intellectual Property Office
UPDATE as of 19 October 2023
The European Patent Office (EPO)’s ‘10-day rule’ will cease to exist from 1 November 2023. From that date, EPO communications will be deemed to be delivered on the same date which they show, rather than 10 days later (as occurs at present). Click here to find out more.
Users of the European patent system will be familiar with the European Patent Office’s (EPO) ’10-day rule’. This is a legal fiction under which official communications from the EPO are normally deemed to have been delivered to the recipient 10 days after the date which they bear. In many cases, it is the end of the 10-day period which is relevant for calculating deadlines, even if the communication was received before then. While not strictly accurate, some users are in the habit of regarding the 10 days as a ‘grace period’ for response to EPO communications.
Although this rule originated as a ‘buffer’ to take into account delays in receiving documents by post, it has continued to be applicable even as the EPO has increasingly moved towards electronic communication. However, this now looks set to change.
At a meeting held by the EPO Committee on patent law on Thursday 12 May 2022, a proposal for ‘dropping’ the 10-day notification rule was preliminarily approved. The proposal still needs to be approved at the Administrative Council meeting in June, but assuming it passes there too, we can expect this change to come into force on 1 February 2023. The proposed change will abolish the 10-day rule regardless of whether communications are delivered electronically or by post.
Essentially, this means that the EPO’s provisions on notification will be brought in line with the PCT, such that the date of a communication will be considered the date of notification and be decisive for determining the expiry of an applicable deadline. As a safeguard against late delivery of communications, the EPO has proposed measures to extend deadlines in cases where delivery of a document is disputed, and it can be established that a document was delivered to the addressee more than seven days after the date it bears. Here the burden will be on the EPO to establish when the document was actually delivered, unlike the PCT, which places the burden on the applicant to prove late receipt.
The proposal to abolish the 10-day rule is part of a wider strategy to support digital transformation in the patent grant procedure. The changes also acknowledge that today’s reality of rapid postal services and electronic communication is very different to the paper-based world that much of the EPC is based on.
Other proposed changes permit transmission of prior art citations to applicants online rather than on paper, acknowledging that such citations might not always be simple written documents but might also be in multimedia formats. The EPO also proposes to rewrite the rules relating to the format of patent application documents in order to relax many of the (now largely obsolete) requirements relating to paper size, quality and formatting, which were designed for documents filed in hard copy. Notably, amendments to the rules on drawings seem to suggest that the EPO might in future allow applicants to submit colour drawings, which would be a very welcome change for applicants in many technical fields. These changes (amongst others in the overall proposal) are proposed to come into force on 1 November 2022, earlier than the proposed notification changes. We will continue to provide updates as more developments at the EPO are made public.
Data provided by Mathys & Squire has featured in an article by The Trademark Lawyer, highlighting a surge in trade marks owned by online fast fashion retailers.
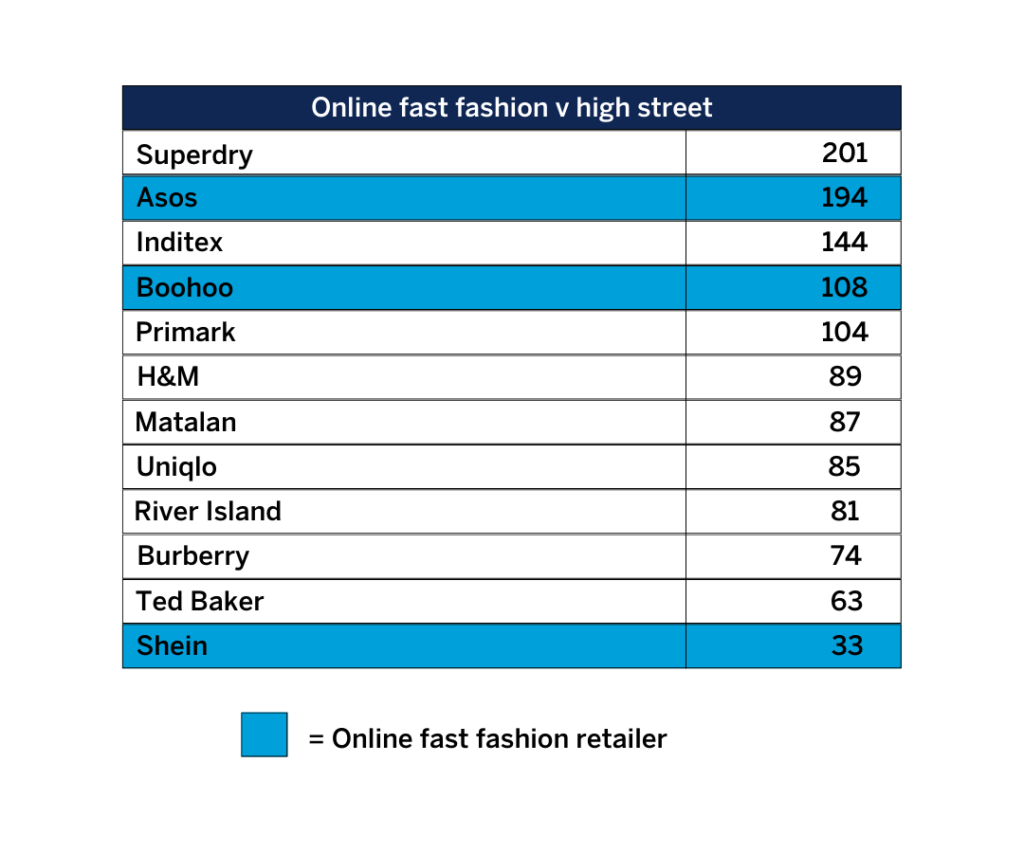
The number of trade marks owned by online fast fashion retailers such as Asos, Boohoo and Shein has increased by 163% from 136 to 358 in the last five years*, shows new research by leading intellectual property law firm Mathys & Squire.
Mathys & Squire says the expansion of the trade mark portfolios reflects the development of fast-moving e-retailers into fully-fledged, multi-brand fashion houses. Key to this strategy is acquiring a formidable portfolio of intellectual property (IP), including both established brands and new trademarks.
Mathys & Squire explains that the size of these e-retailers’ trade mark portfolios is catching up with that of the traditional high street fashion giants. In comparison, the top 10 fashion retailers in the UK by turnover** own a portfolio of 949 trademarks.
Mathys & Squire says expanding their IP portfolios should help fast fashion companies boost their profits, as brand owners typically receive greater profit margins than resellers.
Rebecca Tew, trade mark attorney at Mathys & Squire says: “The growth in the trade mark portfolios of online fashion retailers shows that they are now going toe-to-toe with established high street brands.”
“This expansion of fast fashions brands’ IP portfolios could help boost profit margins in the future.”
Online fast fashion brands such as Asos and Boohoo have begun to rival established high street brands in the size of their IP portfolios. Boohoo now owns 108 UK trade marks, more than Primark (104) and H&M (89), while Asos owns 194 – more than Zara owner Inditex and just shy of Superdry’s 201.Much of this growth comes from acquiring the brands of distressed high street chains such as Karen Millen and Arcadia’s Topshop and Dorothy Perkins.
Mathys & Squire says that there remains value in many legacy brands, including those whose stores have gone into administration.
Rebecca Tew adds: “The way in which online fast fashion retailers have snapped up distressed high street brands highlights that the value of brands can outlast the value of bricks-and-mortar stores. Established brands bring with them name recognition and a loyal following. When combined with the lower overheads of online only retail, the appeal of adding these names to their ‘own brand’ portfolios seems clear.”
“Covid has undoubtedly accelerated the already growing trend towards online shopping, leaving online-only labels in a strong position for future growth.”
*Year ending 31 December, data from Intellectual Property Office
**Largest high street clothing brands by turnover
The 2022 edition of Managing IP’s IP STARS directory has now been released and Mathys & Squire is delighted to have six practitioners named ‘IP Stars’.
We are pleased to congratulate Jane Clark, Paul Cozens and Hazel Ford, who have all been named as ‘Patent stars’, as well as Margaret Arnott, Gary Johnston and Rebecca Tew, who have been ranked ‘Trade mark stars’. Additionally, Philippa Griffin, David Hobson and Andrew White have been featured as ‘Notable practitioners’ in the latest guide.
These IP Stars are senior practitioners who have been recommended or identified as leaders in their firm and/or jurisdiction. Rising star rankings are due to be released in September 2022.
For more information, and to view the rankings in full, visit the IP STARS website here.
Data provided by Mathys & Squire has featured in an article by City A.M. highlighting UK litigation funders’ increased interest in intellectual property cases. Click here to read the article in City A.M.
Eight of the UK’s top 10 litigation funders* are now actively looking to fund intellectual property (IP) cases, says leading intellectual property law firm Mathys & Squire.
Litigation funders pay the legal costs of a litigant’s case in exchange for a share of any damages if the case is won or settled. If the case loses, the litigant owes the funder nothing.
Litigation funding has emerged as a major new asset class for institutional investors over the last decade, meaning there is significant capital to invest in strong intellectual property claims.
IP litigation is a particularly good fit for litigation funding as cases involving the infringement of patents, trade marks and registered designs can be complex, lengthy and international in nature. The cost of pursuing these kinds of claims, often against very deep-pocketed defendants, makes third party funding essential. Of course, the potential for a healthy return, particularly in the US, is another key factor as to why funders find these kind of claims attractive. Even for smaller companies bringing claims, the settlement sums can be significant.
Andreas Wietzke, Partner at Mathys & Squire, says: “Litigation funders see IP cases as a key area to deploy their capital.”
Leading global litigation funder Woodsford says that a particularly attractive growth area for litigation funding is cases of patent infringement by a US corporation against a smaller business such as a UK startup. It is disappointingly common for major tech businesses to meet with startups to discuss their IP under an NDA and then copy their idea, in breach of the NDA.
IP infringement cases in the US can result in very substantial damages being awarded. In 2020, Apple was ordered to pay the California Institute of Technology $838m in a case brought over Wi-Fi technology. In 2016, Inedix Pharmaceuticals won $2.54bn from Gilead Sciences in a dispute over hepatitis C drugs. The year before that, Smartflash Technologies was awarded $533m in damages from Apple related to software patent infringement.
Mitesh Modha, Director at Woodsford says: “Tech giants often assume a small startup won’t have the resources to pursue a potentially expensive and time-consuming patent infringement case. Litigation funders are increasingly enabling David to fight back against Goliath.”
Mathys & Squire says that the availability of litigation funding for UK businesses to pursue IP claims in the US makes it more economically viable to register IP in the US at an early stage. The possibility of pursuing cases using a litigation funder has also led to more UK businesses examining their catalogues of IP for possible instances of infringement by US tech businesses.
Andreas Wietzke says: “Registering your IP in the US can pay for itself many times over if that IP is compromised by an American company.”
“Given the potential for a huge settlement, many companies may have parts of their IP portfolios that have been infringed in the US that are equivalent to ‘Rembrandts in the attic’.”
Small businesses benefit from the increase in litigation funding for IP disputes as it will allow them to challenge the infringement of their IP by larger businesses without having to risk their own cash.
Larger corporations can also benefit from litigation funding through agreements for funders to actively pursue cases on their behalf. This way large businesses can defend their IP portfolios without having to expend resources on actively monitoring infringements themselves.
Andreas Wietzke adds: “For smaller businesses, using a litigation funder means more capital to invest in growth. For bigger businesses, it can allow them to completely outsource IP defence and take it off the senior management agenda.”
“Litigation funding increases the value of IP in particular for smaller companies. For some of them the cooperation with a litigation funder can even be symbiotic, when the startup is interested in an injunction to secure the market and the funder gets a significant part of the financial benefit.”
If a litigation funder wins a patent case on behalf of a company in one EU country then its sets a non-binding precedent in other EU countries – making it easier for the funder to collect damages across the EU. The upcoming Unified Patent Court (UPC) will boost this by offering an EU wide litigation option providing one ruling to be enforced in all participating countries.
*The Top 10 UK litigation funders by assets

(C) Naomi Korn Associates & Mathys & Squire 2022. Some Rights Reserved. These case studies are licensed for reuse under the terms of a Creative Commons Attribution Share Alike Licence.
The following case study has been taken from the “Implications of COVID-19 on SMEs – Reassessing the Role of IP in Multiple Sectors and Industries” report written by Naomi Korn Associates and Mathys & Squire Consulting, November 2021. This case study reviews the impact of the COVID-19 pandemic on SMEs (from early 2020 through to the first quarter of 2021). It focuses on the industries most affected by the crisis and whether intellectual property (IP) and IP management may have helped mitigate its impact through adaptation and change.
Sector overview
The logistics sector has been one of the worst affected industries by the COVID-19 pandemic and continues to suffer setbacks due to renewed lockdowns, travel limitations, and an overall decreased demand. This has required changes to be made by companies which are adapting to a significant consumer demand decrease. Some businesses had to adapt their business proposition and market strategy, to aid the move from physical stores to e-commerce platforms. This pandemic has seen a shift from a transport system focused on the movement of large workforces, to providing a service reducing physical contact, yet still capable of transporting food, personal protective equipment (PPE) and medical supplies.
Analysis
Following the spread of the coronavirus in early 2020, the logistics sector has been severely impacted, with notable reduction across ocean, land, and air freight. At the beginning of the pandemic, Chinese ocean freight saw a 10% reduction in cargo transportation journeys, while there was a 20% drop in air freight, largely due to a sharp drop-off in passenger flights, which often also carry cargo. It is notable that air travel has been heavily impacted due to the social distancing requirements in place and trying to reduce the transmission of the virus. An increase in blank sailing due to decreased demand, delays in air travel due to reduced capacity, and a parallel increase in air freight rates, have all contributed to an increased demand for land and rail freight instead. However, localised lockdowns and travel restrictions have also negatively impacted the feasibility of land freight travel [1].
Overall, these reductions have created cash flow problems for many in the logistics industry, with the continued presence of the virus resulting in tens of thousands of people losing their jobs or being placed on furlough, while planned investment in the industry has either been frozen or cancelled. A recent study has shown a 67%-77% drop in public spending on travel in the US, UK, and Germany in the first half of 2020 [2], with large airlines such as Virgin Australia and Flybe declaring bankruptcy in 2020 due to cash flow limitations from flight cancellations and a substantial drop in consumer demand.
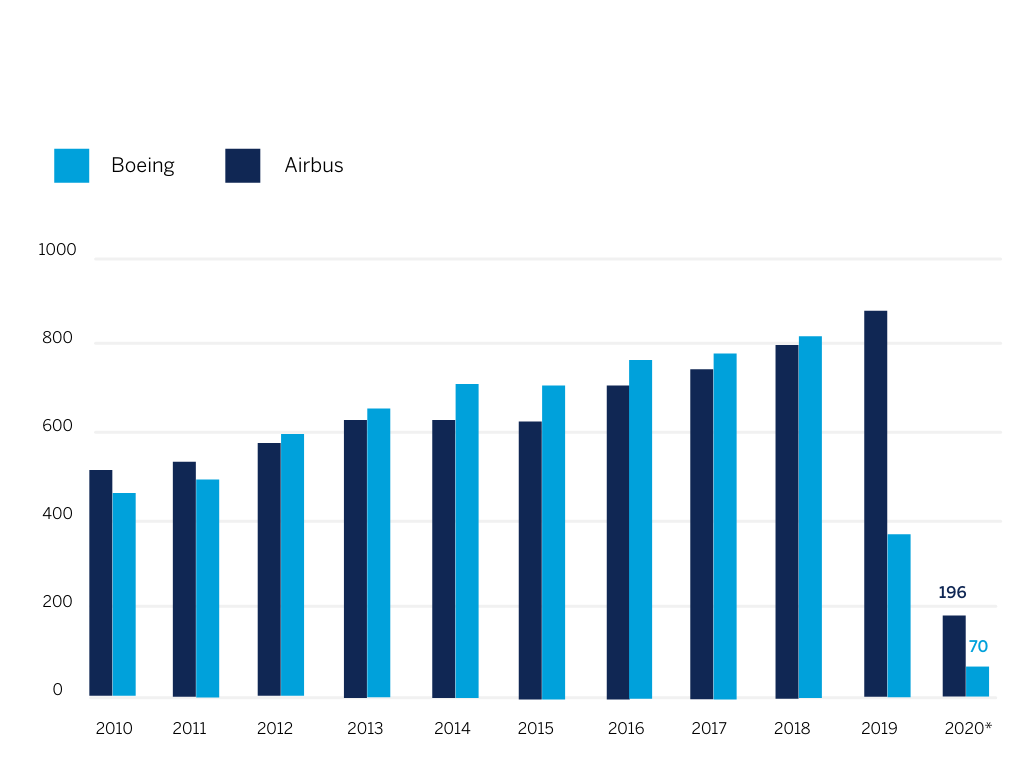
As indicated in Figure 1 below, aerospace manufacturers have also experienced a drastic reduction in demand for new aircrafts. This not only impacts large market players but also the entire supply chain, comprising SMEs from all over the world. The pandemic is expected to cause a contraction in the global air freight forwarding market of between 2%-4% [3]. However, it may also be the case that as normality returns after the pandemic, the aerospace industry starts to reallocate fleets to exclusively serve the air cargo demand, thereby reducing the number of mixed passenger/cargo flights, which are more susceptible to the effects of the pandemic [4].
Crisis critical products [5] such as medical supplies, disinfectant, PPE, and general groceries have seen a significant uptick, although not without challenges in procurement of supply and issues of quality earlier in the pandemic. There has been significant disruption to production value chains, in particular in Asia, as well as trade embargoes and restrictions on movement of certain goods. Consequently, this is likely to increase logistics costs across certain parts of the world for specific goods due to tight regulatory controls and concerns surrounding spreading of the virus.
Business models have also been forced to change, with suppliers increasingly turning to e-commerce platforms, an increased use of electronic cargo visibility and traceability platforms, as well as increased use of automation, robotics, drones and autonomous vehicles [6]. In the longer term, these changes are likely to significantly impact how businesses run and what innovation strategy they develop to further grow. In response to the pandemic and a change in consumer demand, the Chinese company JD developed an Emergency Resources Information Platform (ERIP) for intelligent demand planning, supply risk identification, and visualised tracking for production processes. The ERIP also offers IoT connected automated warehouse management systems and automated guided vehicles to improve efficiency for last-mile logistics, for instance to hospital staff and those under quarantine in Wuhan [7]. Similarly, US based Refraction AI is currently testing last-mile robotics delivery vehicles in Ann Arbor, with vehicles running on the roads, as opposed to footpaths, thereby increasing delivery speeds.
Regarding the response from the logistics industry, there are several lessons that can be learnt about IP management. Companies in the logistics industry have adapted to the digitalisation of the sector by innovating and improving their offering, to match current consumer demands. Thanks to their already existing IP portfolios, as well as new IP assets created through adaptation, companies in the logistics sector have been able to underpin new business and technology models for the protection and exploitation of these assets. Some of the inventions including remote inventory management systems, remote workforce management systems, track and trace systems and digital service delivery. These inventions are protected by copyright, trade secrets and in some cases patents, protecting the IP assets from being infringed on by competitors.
The pandemic has accelerated the digitalisation and generation of robust digital capabilities in the logistics sector and moving forward, these inventions will keep increasing efficiency and reducing costs. As the pandemic begins to come under control, it is the management and protection of these inventions, as well as their importance in contributing to revenues and building value, that management teams must understand.
The Intellectual Property Office of Singapore (IPOS) believes that IP assets and digitalisation go hand in hand and governments and businesses should be seeking to build an ecosystem, focusing on the protection and utilisation of these IP assets, arising from digitalisation. In this context, IPOS has launched an acceleration program designed to expedite the patent granting process to facilitate quicker commercial utilisation of those assets [8]. Recognition of the importance of intangible assets is growing in this sector with a 6.6% increase in transport related patent filings observed by the EPO in 2019, with digital technology and autonomous vehicles patents responsible for much of this growth. In this context, the Chinese logistics firm, JD Logistics has rolled out a fleet of 100 vehicles in Changsu for deliveries home, as well as to hospitals.
However, while the boom in e-commerce has led to significant growth, for many companies it has also led to a marked increase in cases of IP infringement. This raises additional questions surrounding IP responsibility within the logistics supply chain, where traditionally responsibility has been placed on the producer, rather than the retailer or shipper. Moving forward, where patent owners are unable or unwilling to pursue the infringing manufacturer, they may begin to pursue the end retailer or shipper, with significant impacts on the whole supply chain as a likely result. The outcome of current legal cases in the US in this context may potentially spark the revision of supplier agreements from the point of IP protection, as well as opening the supply chain for the analysis of IP risk, thereby having a significant impact on the retail and logistics sectors overall. There is also a need for governments around the world to review their IP enforcement regulations to assist struggling industries, like the logistics industry and support them to enable global economic recovery.
Naomi Korn Associates is one of the UK’s specialists in copyright, data protection and licensing support services.
Mathys & Squire Consulting is an intellectual property consulting team that can support all businesses in capitalising intangible assets.
Naomi Korn Associates and Mathys & Squire Consulting are working in partnership across multiple industries to provide innovative consultancy IP support services.
[1] World Bank Group (2020): The Impact of Covid-19 on Logistics, International Finance Corporation
[2] Statista (2020): Covid-19 Barometer 2020, Statista
[3] Transport Intelligence (2020) Global Freight Forwarding Market Sizing 2020 Covid-19 Impact Analysis, Transport Intelligence
[4] World Bank Group (2020)
[5] Tietze, Vimalnath, Aristodemou, & Molloy (2020): Crisis-Critical Intellectual Property: Findings from the Covid-19 Pandemic, University of Cambridge
[6] World Bank Group (2020)
[7] Capgemini (2020): How digital innovations enabled supply chains to remain operational during the COVID-19 outbreak
[8] IPOS (2021): Acceleration Programmes, https://www.ipos.gov.sg/protect-ip/apply-for-a-patent/accelerated-programmes
We are pleased to announce the appointment of a biotech Partner Iain Armstrong.
Iain has over 20 years’ experience advising clients in the life sciences field, providing practical and pragmatic advice through the entire patent process. He has a particular interest in stem cell technologies, wound healing, and cell therapies (such as CARs), reflecting his background in cell biology. Iain also works extensively on inventions relating to antibody therapeutics, diagnostics, and personalised medicines.
The firm’s new Partner joins Mathys & Squire from IP law firm HGF, where he worked with multiple universities, research organisations and startups across the world. Iain’s practice concentrates primarily on the therapeutic applications of biological inventions, with a particular focus on technologies relating to cell biology.
Martin MacLean, Partner and head of the life sciences team at Mathys & Squire, comments: “I’m absolutely delighted to welcome Iain onboard; it’s a coup for Mathys & Squire to attract a senior attorney of Iain’s calibre. His energy and enthusiasm speak volumes and his track record is exemplary. Iain’s appointment is a major step in furthering Mathys & Squire’s strategic development in the life and medical sciences sectors. His extensive biotech knowledge and legal experience will prove a significant long-term asset to the firm.”
This release has been published in Pro-Manchester, The Patent Lawyer and Juve Patent.