The Royal Swedish Academy of Sciences awarded the 2022 Nobel Prize in the field of Chemistry to K. Barry Sharpless at the Scripps Research Institute, Morten Meldal at the University of Copenhagen, and Carolyn R. Bertozzi at Stanford University, for their roles in the development of click chemistry and bioorthogonal chemistry.
In his paper entitled ‘Click Chemistry: Diverse Chemical Function from a Few Good Reactions’, Sharpless noted that traditional means of reconstructing complex molecules found in nature required the use of complex multi-step reactions which were considered to be less efficient and resulted in the formation of considerable amounts of unwanted byproducts. Sharpless proposed a new approach to the synthesis of complex organic structures aimed at accelerating the rate at which new compositions could be formed. With regards to this new synthetic approach, Sharpless noted “The approach derives from a keen awareness of natures preferred methods of synthesis, but does not seek to emulate them too closely. Nature is a matchless creator of C-C linkages and we propose leaving the tough job of C-C bond synthesis as much as possible to her.” The new method of synthesising complex molecules, referred to as ‘click-chemistry’, was based on a process of forming of heteroatom bridges (C-X-C) between molecular building blocks containing the required carbon structure.
Sharpless stipulated that in order to fall under the term ‘click chemistry’, a reaction must “be modular, wide in scope, give very high yields, generate only inoffensive byproducts that can be removed by nonchromatographic methods, and be stereospecific (but not necessarily enantioselective). The required process characteristics include simple reaction conditions (ideally, the process should be insensitive to oxygen and water), readily available starting materials and reagents, the use of no solvent or a solvent that is benign (such as water) or easily removed, and simple product isolation.”
The ‘click chemistry’ process was further developed when Morten Meldal and Barry Sharpless both independently developed a copper catalysed azide-alkylene cycloaddition reaction, in which one reactant comprises an azide functional group and the other an alkylene functional group. In the presence of a copper (I) catalyst the two reactants form a triazole structure (as shown below). By incorporating these azide/alkylene functional groups, different building blocks may be reacted precisely and efficiently allowing researchers to synthesise more complex organic structures.
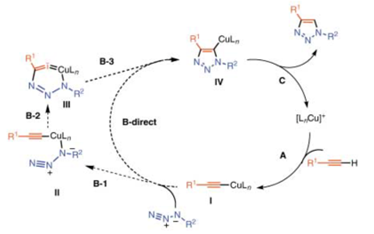
Proposed catalytic cycle for the Cu1-catalysed ligation, taken from “A Stepwise Huisgen Cycloaddition Process: Copper(I)-Catalyzed Regioselective “Ligation” of Azides and Terminal Alkynes” Angew. Chem. Int Ed. 2002, 41 No. 14.
Bertozzi further developed the ‘click chemistry’ process for the purpose of studying glycans on the surface of cells. Previously, it had not been possible to study glycans, sugar-based polymers which play a vital role in our immune responses, on living cells. The bioorthogonol reactions developed by Bertozzi replaced the copper catalyst previously used – which is toxic to organic cells – with cyclooctyne, an 8-membered alkyne ring. Bertozzi discovered that the strain energy within such compounds could be used to drive the ‘click chemistry’ process in the absence a copper catalyst and without disrupting the normal chemistry of the cell.
This research has made a considerable contribution to humankind as it has enabled new drugs and pharmaceutical compositions to be discovered in shorter time periods. New research into cancer treatment has been based on this development. In particular, studies developing clickable antibodies which target different tumours are ongoing.

